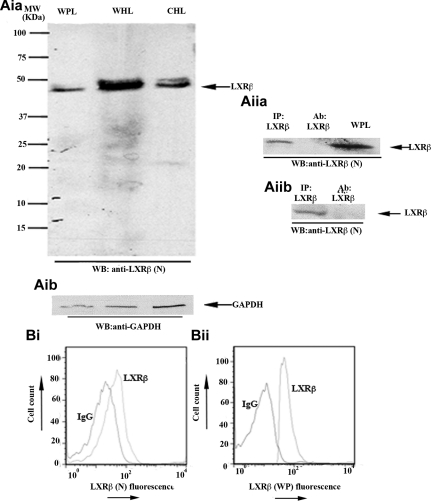
Figure 1.
LXR-β is present in human platelets. (Aia-Aib) Whole platelet lysates (WPL) were immunoblotted for LXR-β (Aia) or glyceraldehyde-3-phosphate dehydrogenase (Aib). Using a mouse monoclonal anti-LXR-β antibody raised against the N-terminus of the protein (LXR-β(N)), a 45-kDa band was detected. Whole HeLa lysates (WHL) and cytosolic HeLa lysates (CHL) were used as positive controls. (Aiia-Aiib) LXR-β was immunoprecipitated (IP) from platelet lysates using antibodies raised against the C-terminus amino acid sequence of LXR-β (Aiia) or the whole protein (Aiib), and IP samples were Western-blotted for LXR-β. WPL and antibodies used for IP were used as positive and negative controls, respectively. (B) Human platelets were stained with mouse (Bi) or rabbit (Bii) anti-LXR-β antibodies or the same amount of IgG control, followed by equivalent PE-conjugated secondary antibodies and analyzed by flow cytometry. A shift in the fluorescence profile was observed when anti-LXR-β antibodies were used. MW indicates molecular weight.