To the editor:
Leung et al demonstrated the potential importance of the tetraspanin CD91 in regulating migration, adhesion, and homing of human umbilical cord blood (UCB) CD34+ hematopoietic stem and progenitor cells.2 Using a neutralizing antibody to CD9 they inhibited marrow and splenic homing of these cells in sublethally irradiated NOD/SCID mice. They also showed inferior homing of CD34+CD9− cells compared with total CD34+ cells.
Our laboratory has also been interested in defining the role of a tetraspanin-containing polarized membrane domain in the interaction of primitive hematopoietic cells with osteoblasts, critical components of the hematopoietic stem cell niche.3 We have investigated whether increasing expression of tetraspanins such as CD9 might increase homing in the niche. We and the Leung group have found that exposure of CD34+ cells to a protein kinase C ϵ agonist, ingenol 3,20-dibenzoate (IDB) can increase CD9 expression. In our study, in contrast to Leung's results, we found that 4 hours' exposure to IDB was insufficient time to increase the fraction of CD34+ cells expressing CD9 expression in either mobilized peripheral blood (MPB) or cord blood (CB) CD34+ cells. There was also no change in CXCR4 expression in the IDB versus control cells at 4 hours.
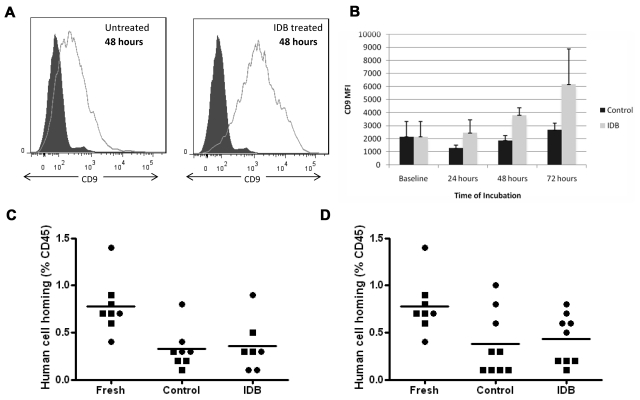
In 3 experiments, we tested whether longer incubation times with IDB could induce CD9 up-regulation in MPB CD34+ cells. When cells were incubated for 48 hours, the mean fluorescence intensity (MFI) for CD9 increased on the IDB-treated cells compared with the untreated cells as shown in a representative experiment (Figure 1A) and summarized in Figure 1B (P = .009; n = 3). An increase in CD9 MFI was also observed on IDB-treated cells after 72 hours in culture and the difference approached significance compared with the untreated group (P = .094; n = 3; Figure 1A-B). Expression of other molecules known to be important for adhesion and homing, including VLA-4 and CXCR-4 showed no significant modulation in MFI or fraction of CD34+ cells expressing each molecule after incubation of MPB CD34+ cells for 24 to 72 hours in IDB compared with controls (data not shown).
Figure 1.
Incubation of human mobilized peripheral blood (MPB) CD34+ cells. Incubation of human mobilized peripheral blood (MPB) CD34+ cells for 48 to 72 hours with IDB up-regulates CD9 expression compared with MPB CD34+ cells incubated without IDB, but homing IDB-treated cells in NSG mice is not improved. (A) Flow cytometric analysis of CD9 expression on human MPB CD34+ cells cultured for 48 hours in the absence (left panel) or presence (right panel) of IDB. (B) Summary of the CD9 MFI of human MPB CD34+ cells at baseline and after culture in the presence or absence of IDB for 24, 48, or 72 hours. (C) Summary of homing studies after 48 hours of incubation with IDB. Human MPB CD34+ cells were exposed to IDB for 48 hours before transplantation into NSG mice; cells in the control group were cultured without IDB. In both groups homing of the human cells to the mouse bone marrow was measured by flow cytometry using human CD45 antibodies 18 hours after transplantation (■, Donor 1; ●, donor 2). (D) Summary of homing studies after 72 hours of incubation with IDB. Human MPB CD34+ cells were exposed to IDB for 72 hours before transplantation into NSG mice; cells in the control group were cultured without IDB. In both groups, homing of the human cells to the mouse bone marrow was measured by flow cytometry using human CD45 antibodies 18 hours after transplantation (■, Donor 1; ●, donor 2).
To determine whether the increase in CD9 expression would affect homing, we transplanted NOD/SCID γ (NSG) mice with cultured MPB CD34+ cells (1 × 106 cells/mouse, n = 3-5 mice/group) incubated for 48 and 72 hours with or without IDB in 2 separate experiments using different donors. NIH Animal Care and Use Committee approval was given. Mice were killed 18 hours after transplantation, and the homing of human cells to the murine bone marrow was analyzed by flow cytometry for human CD45+ cells. Compared with MPB CD34+ cells cultured without IDB, no statistically significant improvement in marrow homing was seen in mice transplanted with cells exposed to IDB for 48 hours (P = .8; Figure 1C) or 72 hours (P = .97; Figure 1D). In a separate experiment, no transplanted CD45+ cells were detected in blood or splenic tissue (data not shown).
Despite differences in the density of CD9 per cell, exposure to IDB did not result in improved homing in NSG mice. This finding was not because of down-regulation of other surface adhesion receptors essential for hematopoietic stem cell homing. An up-regulation of CD9 did not result in cells lodging in splenic tissue preventing them from reaching the bone marrow. We conclude that while decreasing the percentage of CD34+CD9+ cells inhibits homing as shown by Leung et al,1 basal cell–surface CD9 density appears to be sufficient for maximal homing; therefore, CD9 modulation with IDB unfortunately lacks promise for improving homing and engraftment.
Authorship
Contribution: R.D. conducted experiments and wrote the paper; A.D. conducted experiments; F.R. provided expertise; C.E.D. provided expertise and wrote the paper; and A.L. provided expertise, conducted experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Cynthia E. Dunbar, NHLBI, Bldg 10/4E-5132, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: dunbarc@nhlbi.nih.gov.
References
- 1.Yáñez-Mó M, Barreiro O, Gordon-Alonso M, et al. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19(9):434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Leung KT, Chan KY, Ng PC, et al. The tetraspanin CD9 regulates migration, adhesion and homing of human cord blood CD34+ hematopoietic stem and progenitor cells. Blood. 2011;117(6):1840–1850. doi: 10.1182/blood-2010-04-281329. [DOI] [PubMed] [Google Scholar]
- 3.Gillette JM, Larochelle A, Dunbar CE, Lippencott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11(3):303–311. doi: 10.1038/ncb1838. [DOI] [PMC free article] [PubMed] [Google Scholar]