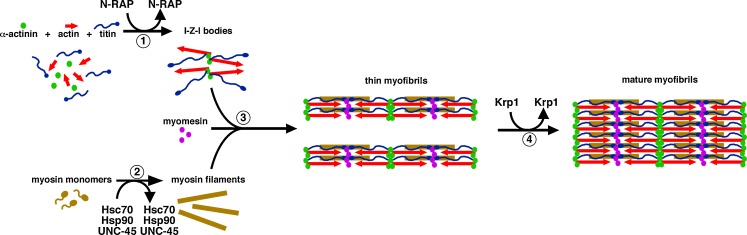
Fig. 1.
A putative pathway for myofibril assembly highlighting the role of transiently associated proteins in organizing the major structural components (modified from Greenberg et al. 2008). (1) N-RAP promotes assembly of the I-Z-I structures containing actin, α-actinin, and N-terminal titin. (2) Myosin filaments form separately, with appropriate folding and assembly promoted by the Hsc70 and Hsp90 chaperone proteins and the UNC-45 co-chaperone. (3) Titin associates with the myosin filaments along their length, helping to integrate the thick filaments with the I-Z-I structures. In addition, myomesin interacts with titin and myosin to crosslink the array of myosin filaments at the center of the sarcomere. This gives rise to thin myofibrils. (4) Finally, Krp1 promotes the lateral fusion of thin myofibrils to form mature myofibrils