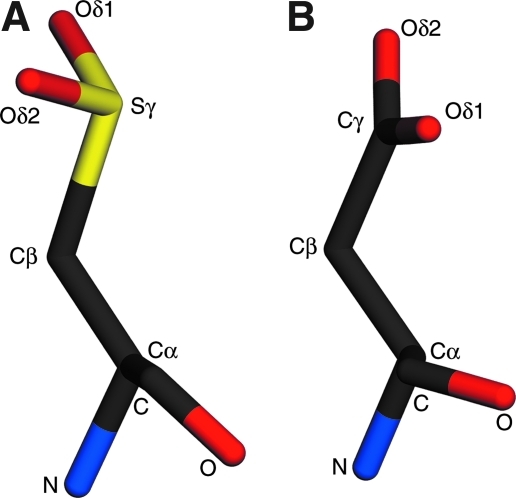
FIG. 3.
Aspartate is not a good structural mimic for cysteine-sulfinate. In both panels, all atoms are labeled. (A) A stick representation of cysteine-sulfinate, illustrating that the lone pair of electrons on the Sγ atom results in a bent geometry for the oxygen atoms (Oδ1 and Oδ2) in relation to the sulfur atom. (B) The different electronic configuration of the Cγ atom in aspartate results in sp2 hybridization and a planar arrangement of the oxygen atoms with Cγ atom. This geometric difference prevents aspartate from satisfying the same set of hydrogen bonds that are made by Cys106-SO2− (Fig. 2), which may help explain why the C106D DJ-1 mutant is not functional.