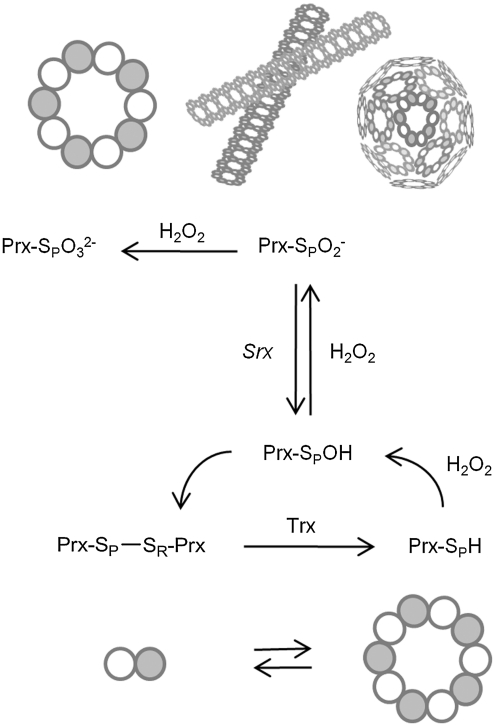
FIG. 1.
Typical 2-Cys peroxiredoxin catalytic cycle and hyperoxidation. Low levels of H2O2 are reduced by Prx through a pair of essential Cys residues, Cys-SPH and Cys-SRH. The sulfenic acid intermediate (Cys-SPOH) reacts with the Cys-SRH residue to form an intermolecular disulfide bond, which is subsequently reduced by thioredoxin. During this process, the Prx molecules alternate between dimeric and decameric states. The reduced, decameric form of the protein is the most reactive with H2O2 (51, 73, 75). As the level of H2O2 increases, eukaryotic Prxs can react with a second H2O2 molecule to form the sulfinic acid form (CysSPO2-) and, as a result, are inactivated. This hyperoxidation stabilizes the decameric state of the Prx molecule and can lead to the formation of filamentous and spherical, high-molecular-weight species; depicted schematically here. The molecular details of these interactions are unknown. Further oxidation of the Prx molecule to the Cys sulfonic acid form (CysSPO32-) can occur. Srx, however, can only reduce the CysSPO2- moiety.