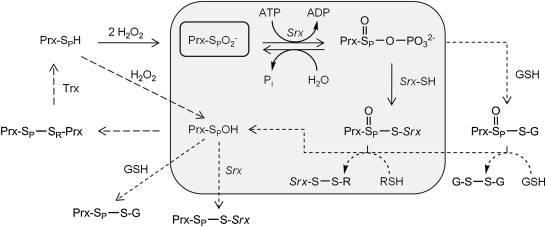
FIG. 3.
Sulfiredoxin reaction mechanism and intermediates. The original mechanism, based on the analysis of S. cerevisiae Srx (gray shading), relies on the formation of sulfinic phosphoryl ester (Cys-SPO2PO32-) and a thiosulfinate intermediate (Prx-SPO-S-Srx) between the Srx and Prx molecules (6). Structural and biochemical data support the direct formation of the former intermediate (see text for details). The Srx-Prx thiosulfinate intermediate has been confirmed for the yeast and human enzyme systems (33, 55). On reduction of this thiosulfinate with GSH or Trx (R-SH), the repaired Prx molecule (Prx-SPOH) can return to the Prx catalytic cycle (long dashed lines). A recent study showed that yeast Srx, which contains an additional Cys residue within a loop insertion (Fig. 2; also see the regions highlighted in green in Fig. 4), can resolve the Srx-Prx thiosulfinate through the formation of an intramolecular disulfide bond [Srx-(S-S)] (56). Alternative reaction paths and intermediates between Srx, Prx, and GSH (short dashed lines and arrows) remain to be investigated.