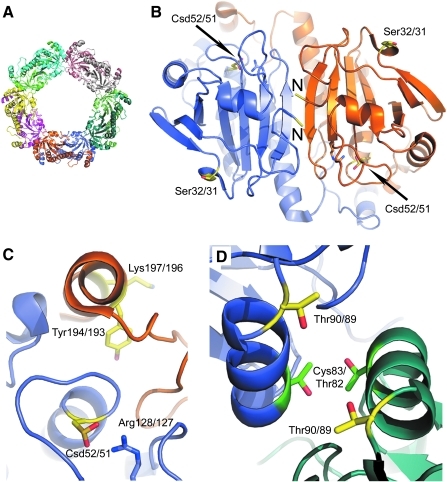
FIG. 6.
Sites of covalent modification for human PrxI and PrxII. (A) The hyperoxidized PrxII decamer with each monomer represented in a different color (PDB code 1QMV) (58). (B) Close-up of one Prx dimer highlighting the monomer–monomer interface near the N-termini, labeled N. Sites of covalent modification in all panels are colored yellow. The Cys-SPH residue is present in the sulfinic acid form (Csd). Numbering scheme used: PrxI residue number/PrxII residue number. Ser32/31 and the N-termini are located on the back of the Prx dimer away from the Prx active sites. (C) Close-up of the active site. Tyr194/193, part of the YF motif, and Lys197/196 are proximal to the peroxidatic Cys residue. (D) The dimer–dimer interface. Thr90/89 can be phosphorylated. For reference, the mutation of Thr82/Cys83 to Glu (green) results in the disruption of the decamer into its dimeric constituents (28). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).