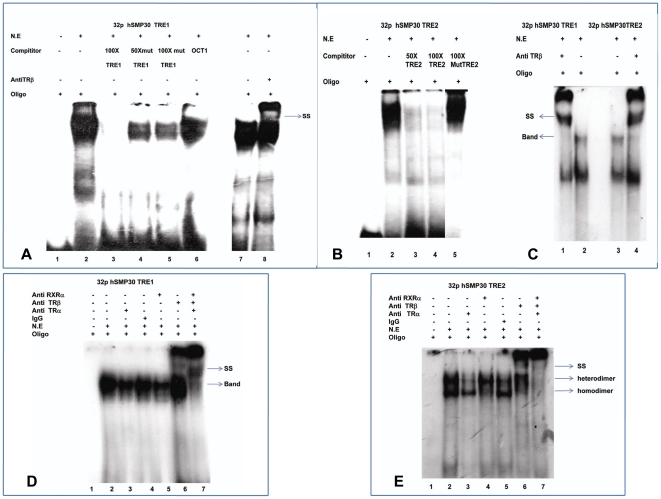
Figure 2. Identification of high affinity TR binding sites within hSMP30 Promoter.
(A) Electrophoretic mobility shift assay for hSMP30 TRE1 to confirm the binding of TR. lane 1, labeled hSMP30 TRE1; lane 2, 7 are labeled hSMP30 TRE1 with 10 µg of Nuclear Extract (N.E.); lane 3–6 describe competition with 100 fold molar excess of self oligo, 50 and 100fold molar excess of mut TRE1 oligo, nonspecific oligo and lane 8 antibody shift with TRβ antibody. (B) Electrophoretic mobility shift assay for hSMP30 TRE2 to confirm the binding of TR. Lane 1, labeled hSMP30 TRE2 with 10 µg nuclear extract (N.E); Lane 2–4 describe competition with 50 and 100 fold molar excess of self oligo and mutated TRE2 oligo. (C) As antibody shift was not distinct for short run i.e. for 1 hour, we did long run for two hours, then we got distinct antibody shift in fig. 2C. Lane 2 and 3 are labeled hSMP30 TRE1 and TRE2 with 10 µg N.E; Lane 1 and 4 are antibody shift with TRβ antibody. (D, E) To confirm the binding of TRβ is specific to hSMP30 TRE1 and TRE2, we did electrophoretic mobility shift assay in presence of control IgG, TRα, TRβ and RXRα antibodies. Lane 1 labeled hSMP30 TRE1 and TRE2; lane 2 labeled hSMP30 TRE1 and TRE2 in presence of 10 µg of N.E of MCF-7 cells - transfected with TRα, TRβ and RXRα expression vectors; lane 3, 4, 5, 6 antibody shift with TRα, normal Rabbit IgG, RXRα and TRβ respectively; lane 7 labeled hSMP30 TRE1 and TRE2 in presence of 10 µg of N.E incubated with TRα, TRβ and RXRα antibodies in a single reaction. Arrows indicate retarded and supershifted complexes.