Abstract
Using the most comprehensive approach to selecting polymorphisms to date, we sought to examine whether time to recurrence in ovarian cancer was associated with common inherited variation in eight genes involved in drug metabolism, multi-drug resistance, or DNA repair, namely ABCB1, CYP2C8, CYP3A4, ERCC1, ERCC2, GSTM1, XPC, and XRCC1. Invasive epithelial ovarian cancer patients (N=445) seen at the Mayo Clinic from 1999 to 2009 with 275 observed recurrences or deaths were analyzed at 94 SNPs in these candidate genes. Cox regression was used to estimate hazard ratios and 95% confidence intervals for each single nucleotide polymorphism (SNP) and outcome (defined as time to recurrence or death). Analyses were conducted at the gene level and on case subsets defined by histopathology and chemotherapeutic agent. At ABCB1, minor alleles at several SNPs were associated with outcome, with the most significant being the intronic SNP rs12334183 (HR=0.65, 95% Cl 0.51-0.83; p=0.0005). Overall variation in ABCB1 was predictive of outcome as well (p=0.003). At ERCC2, minor alleles at several SNPs were associated with outcome among women with high-grade serous disease (e.g., rs238417, HR 0.74, 95% Cl 0.59-0.92; p=0.006). No associations with outcome were observed in GSTM1, CYP2C8, CYP3A4, ERCC1, XPC, or XRCC1. In summary, inherited variation in ABCB1 and ERCC2 was associated with outcome in patients with ovarian cancer seen at the Mayo Clinic. As the associated SNPs have not been studied previously in ovarian cancer, these findings suggest novel sites of variation which may, in part, explain the range of treatment responses seen in this disease.
Keywords: Ovarian cancer, drug-related variants, metabolism
Introduction
Ovarian cancer is the fifth leading cause of cancer death among women in the United States, with an estimated 21,880 new cases and 13,850 deaths in 2010 [1]. Paclitaxel (Taxol) or docetaxel (Taxotere) and platinum-based chemotherapy (either Cisplatin or Carboplatin) are standard therapies after surgical debulking. Unfortunately, even with modern chemotherapy, most patients with advanced disease relapse and die of ovarian cancer with five-year survival lingering around 30% [2, 3]. Patients surviving longer may have inherited variants in genes related to metabolism of or resistance to these chemotherapy agents.
Taxol resistance may be related to multi-drug resistance genes (ATP binding cassette transporter genes) such as ABCB1, ABCB2, and ABCG2 which encode phosphoglycoprotein (pgp), a transmembrane transporter that leads to energy-dependent efflux of several drugs including taxol. The Australian Ovarian Cancer Study analyzed SNPs in ABCB1 in patients treated with paclitaxel and carboplatinum and found a decreased risk of recurrence associated with certain SNPs in optimally-debulked patients; however, this finding was not seen in a larger independent validation set (SC0TR0C1) [4]. Selected polymorphisms in multidrug resistant genes ABCG2, ABCB1, and ABCC2 were also analyzed in 385 women in the Gynecologic Oncology Group (GOG) studies 172 or 182 wherein women received paclitaxel and platinum chemotherapy after optimal debulking of stage III disease [5]. A statistically-significant decrease in time to cancer progression was observed in women with polymorphisms in ABCG2, but not in ABCB1, or ABCC2 [5]. Other key genes are CYP2C8, which catalyzes hydroxylation of paclitaxel at the 6th position to form 6-alpha paclitaxel, and CYP3A4, which hydroxy-lates the C13 position to form metabolites with 10-fold to 40-fold less cytotoxicity [6, 7]. Polymorphisms in CYP2C8 have also been correlated in vitro with clearance of paclitaxel [8]. However, no associations were seen in the largest ovarian cancer study, SCOTROC1, of 914 cases and 16 polymorphisms in genes related to response and toxicities to taxanes (ABCB1, ABCC1, ABCC2, ABCG2, CDKN1A, CYP1B1, CYP2C8, CYP3A4, CYP3A5, MAPT, and TP53) [9].
Although mechanisms of platinum resistance are not well understood, it has been postulated that abnormalities in DNA binding or repair mechanisms may cause this resistance. Several studies suggest that in cancers where platinum-based chemotherapy is effective, DNA repair polymorphisms predict survival [10-14]. Studies of other cancers show associations between outcome of head and neck squamous cell carcinoma and polymorphisms in XPD (Xeroderma pigmentosa group D), XPC (Xeroderma pigmentosa group C), XRCC1 (X-ray repair cross-complementation group 1), and ERCC1 (Excision repair cross-complementation group 1) genes [11]; lung cancer and polymorphisms in ERCC1 [12], XPD, and XPC [14]; and advanced platinum-treated colorectal cancers and ERCC1 polymorphisms [13]. In ovarian cancer, large population-based studies have suggested that polymorphisms in DNA repair [9, 15] or the cell cycle pathway [16] were not important in determining survival in ovarian cancer patients. The GOG has also studied ERCC1 polymorphisms and found associations with progression free survival and overall survival in advanced ovarian cancer patients treated with paclitaxel and platinum [17]. ERCC2 (Excision repair cross -complementation group 2) is also involved in the nucleotide excision repair mechanism and SNPs in this gene have been studied in the SCO-TROC1 trial and also in the Korean population without significant associations to platinum response, toxicity, or survival [9, 18]. SCOTROC1 examined nine polymorphisms in genes related to response and toxicities to taxanes platinum (ABCC2, ABCG2, ERCC1, ERCC2, MPO, and XRCC1) and found no association [9].
Finally, GST (glutathione S transferase) catalyzes the conjugation of various electrophilic compounds, including paclitaxel and platinum and abnormalities in this gene may cause chemotherapy resistance. A previous study of GST polymorphisms found that mean survival was longer for the GSTM1 null type (40.5 months v. 33.5 months, p=0.006) or carriers of non-GSTM1/GSTT1 common genotypes in ovarian cancer patients (55.4 months vs. 30.7 months, p=0.009) [19]. The SCOTROC1 study examined two polymorphisms in GSTP1 and found no association with response or toxicities [9].
Thus, previous studies yielded discrepant results and were hindered with the inclusion of only small numbers of putatively-functional polymorphisms per gene. We, therefore, aimed to more comprehensively assess variation in the key genes ABCB1, CYP2C8, CYP3A4, ERCC1, ERCC2, GSTM1, XPC, and XRCC1 by evaluating all known underlying common polymorphisms and selecting a maximally informative set to study in relation to time to recurrence in over 440 invasive epithelial ovarian cancer patients at the Mayo Clinic.
Methods
Study participants
Recruitment of patients from Mayo Clinic's gynecologic surgery and medical oncology departments, including administration of questionnaires and venipuncture, used established protocols approved by the Institutional Review Board [20]. All participants gave written informed consent and included women aged 20 years or older living in Minnesota, Iowa, Wisconsin, Illinois, North Dakota, or South Dakota and ascertained within one year of a diagnosis of pathologically-confirmed primary invasive epithelial ovarian cancer. A total of 480 invasive ovarian cancer patients recruited between December 14, 1999 and May 28, 2009 and diagnosed within the preceding year (median time from recruitment to diagnosis was two days) were enrolled; 13 sequence-confirmed BRCA1 or BRCA2 mutation carriers were then excluded. Data on clinical features of disease including histology, surgical outcomes, and chemotherapy were abstracted by an experienced research nurse and reviewed by gynecologic and medical oncology clinicians. Recurrence data were obtained via the Mayo Clinic computerized medical record, defining recurrence date as the date of initiation of second-line therapy. Vital status was obtained from several sources including the National Death Index, the Mayo Clinic computerized medical record, and the Mayo Clinic Cancer Registry, which follows patients annually for overall survival who were diagnosed or received initial treatment at Mayo Clinic. To confirm dates of death, 151 death certificates were obtained and were found to be 96.0% concordant with dates obtained via the mechanisms described above; the median discrepancies in dates for six discrepant patients was two days. Follow-up data were obtained through March 2, 2010; 17 cases were lost to follow-up before 2008. DNA was extracted from 10 to 15 mL fresh peripheral blood using the Gentra Auto-Pure LS Purgene salting out methodology (Gentra, Minneapolis, MN), bar-coded to ensure accurate processing, and stored at −20°C.
Genes and polymorphisms
Details on genes and single nucleotide polymorphisms (SNPs) are provided in Table 1 and additional information will be available on request from the corresponding author. For each gene, we selected tagSNPs within 5 kb upstream and downstream with minor allele frequency (MAF) ≥0.05 based on pair-wise linkage disequilibrium (LD, r2≥0.9) [21] among Europeans in HapMap Consortium's release 22 [22], or, for CYP3A4, NIEHS SNPs [23] which provided data on more SNPs for this gene than HapMap. After grouping SNPs into LD bins, tagSNPs were evaluated for compatibility with a 96-SNP Illumina VeraCode Assay, and prioritized by VeraCode design score; two tagSNPs were chosen from bins with ten or more SNPs. One XRCC1 tagSNP was incompatible with genotyping (design score <0.4); thus, we re-binned XRCC1 excluding this SNP. Ninetyeight tagSNPs were selected; therefore, two ABCB1 SNPs (rs9282564 and rs2888599) which had been previously genotyped in a majority of these patients [24] (Goode EL, Chenevix -Trench G, Hartmann LC, Fridley BL, et al: Assessment of hepatocyte growth factor in ovarian cancer mortality, submitted) were excluded leaving 96 SNPs on a custom genotype array (additional information will be available on request from the corresponding author).
Table 1.
Gene Information
| Name | GeneID | RefSeq | Chr | Str | Start (bp) | Size (bp) | Description | tagSNP Source | Bin Coverage | N SNPs Analyzed (Attempted) |
|---|---|---|---|---|---|---|---|---|---|---|
| GSTM1 | 2944 | NM_000561.3 | 1 | + | 110,031,965 | 5,925 | Glutathione S-transferase M1 | HapMap | 100% | 1 (1) |
| XPC | 7508 | NM_004628.4 | 3 | + | 14,161,648 | 33,495 | Xeroderma pigmentosum, complementation group C | HapMap | 85% | 11 (11) |
| ABCB1 | 5243 | NM_000927.3 | 7 | + | 86,970,884 | 209,616 | ATP-binding cassette, sub-family B (MDR/TAP), member 1 | HapMap | 91% | 33 |
| CYP3A4 | 1576 | NM_017460.3 | 7 | + | 99,192,540 | 27,204 | Cytochrome P450, family 3, subfamily A, polypeptide 4 | NIEHS SNPs | 63% | 5 (5) |
| CYP2C8 | 1558 | NM_000770.3 | 10 | - | 96,786,519 | 32,725 | Cytochrome P450, family 2, subfamily C, polypeptide 8 | HapMap | 92% | 14 (14) |
| XRCC1 | 7515 | NM_006297.2 | 19 | + | 48,739,304 | 32,251 | X-ray repair complementing defective repair in Chinese hamster cells 1 | HapMap | 100% | 16 (16) |
| ERCC2 | 2068 | NM_000400.3 | 19 | + | 50,546,686 | 18,983 | Excision repair cross-complementing rodent repair deficiency, complementation group 2 (xeroderma pigmentosum D) | HapMap | 85% | 11 (11) |
| ERCC1 | 2067 | NM_001983.2 | 19 | + | 50,604,712 | 14,305 | Excision repair cross-complementing rodent repair deficiency, complementation group 1 (includes overlapping antisense sequence) | HapMap | 60% | 3 (5) |
| Total | 94 (96) | |||||||||
Genome build 36.3; Refseq Release 29; chr, chromosome, str, strand, bp, base pairs; N analyzed SNPs excludes two (2%) which failed genotyping; sorted by chromosomal position; bin coverage calculated as number of bins with one or more tagSNPs successfully genotyped divided by the number of total bins (SNPs failed either due to incompatibility with assay design or to genotype failure).
Genotyping and exclusions
Genotyping of 467 participants, 30 duplicates, and 17 laboratory standards including CEU trio data was performed at the Mayo Clinic using an Illumina Veracode Assay [25]. Following DNA activation, incubation, amplification, and automated genotype calling [25, 26], quality control exclusions were performed using all data. Of the 467 participants genotyped, two samples failed, and four samples were excluded based on call rates <95%. Additional participants were excluded based on unknown tumor stage (N=4), self-reported non-Caucasian race (N=2), and non-epithelial ovarian cancer discovered upon additional pathology review (N=10). Of the 96 SNPs, two SNPs (2%) failed (ERCC1 rs11615 and ERCC1 rs2298881). Concordance across duplicates was 98.7%, no Mendelian errors were observed among the CEU trio, and consistency of 93 replicates of HapMap CEU sample NA10859 was 100%. We assessed departures from Hardy Weinberg equilibrium (HWE) with a Pearson goodness-of-fit test or, for SNPs with a MAF<0.05, a Fisher exact test. SNPs were screened for HWE p-values<0.0001, MAF<0.01, and call rate <95%; however, no exclusions were necessary leaving 94 SNPs and 445 participants for statistical analysis.
Genetic association analysis
We used Cox proportional hazards regression [27] to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for association with time-to-recurrence or death. For each SNP, a primary association using an ordinal coding (i.e., 0, 1, 2 copies of minor allele) was tested (analogous to the Armitage test for trend for binary endpoints) [28], and HRs with 95% CIs were estimated per-allele. Time of analysis was from date of diagnosis to recurrence (initiation of second-line therapy) or death, accounting for left truncation as patients were enrolled subsequent to initial diagnosis. Subjects alive and recurrence-free were censored at last follow-up. Analyses were performed on all patients as well as on patients with high-grade serous disease only. In addition, analyses at ABCB1, CYP3A4, and CYP2D8, were restricted to patients treated with taxane agents (taxol/paclitaxel or taxotere/ docetaxel); analyses at XPC, ERCC1, ERCC2, and XRCC1 were restricted to patients treated with platinum agents (cisplatin or carboplatin); analyses at GSTM1 were restricted to patients treated with either taxane or platinum agents. Primary analyses were unadjusted for covariates; however, sensitivity analyses were also performed adjusted for age, stage, grade, presence of ascites, and degree of debulking (factors associated with outcome at p<0.05) to evaluate whether observed SNP associations acted via an influence on these clinical factors. Gene-level tests were conducted using principal component analysis in a manner similar to that described previously [29]; components that explained 90% of SNP variance within each gene were included as predictors in Cox models and simultaneously tested for association using a multiple degree-of-freedom likelihood ratio test. Gene effects were also examined using analyses of haplotypes with estimated frequency ≥0.05 [30]. Due to the exploratory nature of all analyses, associations were considered statistically significant at p≤0.01. Plots of LD displaying r2 for each gene were created using Haploview version 4.2 [31].
Results
Characteristics of 445 successfully-genotyped invasive epithelial ovarian cancer patients are shown in Table 2. The majority of patients had tumors of serous histology (66%) and was diagnosed at stage III or IV (74%). Most patients underwent primary debulking surgery at Mayo Clinic (93%) with ≤1 cm tumor remaining (85%) and had presence of ascites (63%) and positive cytology (64%). A total of 275 patients (62%) recurred or died during the study period including 191 high-grade serous cases; among 170 non-recurring living patients, median follow-up time was 3.2 years (range, 0.1 to 8.9 years).
Table 2.
Characteristics of 445 ovarian cancer patients
| Clinical Factor | N Cases (%) |
|---|---|
| Recurrence status | |
| Recurred or deceased | 275 (62%) |
| No recurrence | 170 (38%) |
| Histologic Sub-type | |
| Serous | 289(66%) |
| Mucinous | 19 (4%) |
| Endometrioid | 74(17%) |
| Clear cell | 29 (7%) |
| Mixed epithelial | 27 (6%) |
| Age at diagnosis, yrs | |
| <50 | 78(18%) |
| 50-59 | 114 (26%) |
| 60-69 | 119(27%) |
| 70+ | 134 (30%) |
| Stage | |
| I | 85(19%) |
| II | 32 (7%) |
| III | 251 (56%) |
| IV | 77(17%) |
| Grade | |
| 1-2 | 70(16%) |
| 3 | 207 (48%) |
| 4 | 151 (35%) |
| Tumor volume following surgery | |
| Optimal, <_ 1 cm remaining | 363 (85%) |
| Suboptimal, >_ 1 cm remaining | 65(15%) |
| Ascites | |
| Yes | 215 (63%) |
| No | 125 (37%) |
| Chemotherapy | |
| Yes | 404 (91%) |
| No/Unknown | 41 (9%) |
| Chemotherapeutic Agent | |
| Platinum and taxane | 353 (91%) |
| Platinum only | 17 (4%) |
| Taxane only | 4 (1%) |
| Neither platinum or taxane | 12 (3%) |
Numbers may not add to 445 due to missing data; key demographic and clinical factors shown.
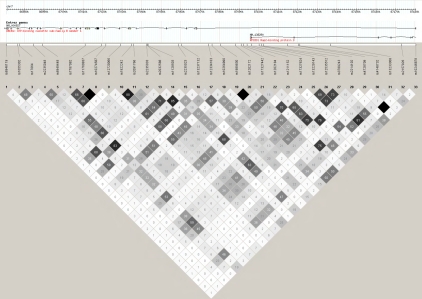
At ABCB1, minor alleles at several SNPs were associated with outcome, with the most statistically significant being the intronic rs12334183 tagging the intronic rs2235015, rs10260862, and rs10280623 (HR 0.65, 95% Cl 0.51-0.83; p=0.0005; Table 3). We examined the impact of adjustment for clinical variables associated with outcome (age, stage, grade, presence of ascites, and degree of debulking) and found some attenuation in risk estimates (e.g., rs12334183 HR 0.74, 95% Cl 0.57-0.95; p=0.02). This suggests that although SNPs may impact outcome via an influence on these clinical features, several ABCB1 SNPs remained predictors of outcome at p<0.05 above and beyond clinical features. LD among the ABCB1 SNPs studied was variable (Figure 1); thus, many of the associations with p<0.05 were at only loosely-correlated SNPs; for example r2 between rs2235023 and rs12334183 was 0.28. Accounting for residual LD, we collapsed the 33 genotyped ABCB1 SNPs into nine orthogonal principal components and assessed whether these were associated with outcome. Indeed, these components were predictive of outcome (p=0.003; Table 4). We also assessed association among a subset of 365 cases known to be treated with taxol/paclitaxel, taxotere, or do-cetaxel (82%) and found that most associations remained despite the reduced sample size (Table 3). Finally, analysis of ABCB1 among a subset of 262 high-grade serous cases showed the same trends in SNP association, but didn't suggest a sub-type-specific association.
Table 3.
Ovarian cancer time to recurrence per-allele hazard ratios (HRs) and 95% confidence intervals (95%CI) for SNPs in ABCB1 and ERCC2
| All Sub-types (N=445) | All Sub-types on Taxane (N=365) | |||||
|---|---|---|---|---|---|---|
| Gene | rsid | kb to next | HR (95% CI) | p | HR (95% CI) | p |
| ABCB1 | rs6946119 | 4.1 | 1.02 (0.84 -1.25) | 0.83 | 0.95(0.76-1.19) | 0.66 |
| rs1055302 | 0.6 | 0.84(0.64-1.10) | 0.20 | 0.82(0.61-1.11) | 0.20 | |
| rs17064 | 5.0 | 0.82(0.57-1.17) | 0.28 | 0.75(0.50-1.13) | 0.17 | |
| rs2235048 | 3.3 | 1.05 (0.89 -1.24) | 0.54 | 1.06(0.88-1.27) | 0.56 | |
| rs6949448 | 15.2 | 0.95(0.80-1.12) | 0.52 | 0.95(0.79-1.15) | 0.62 | |
| rs7787O82 | 6.0 | 0.84 (0.66 -1.06) | 0.14 | 0.90(0.69-1.16) | 0.41 | |
| rs1176O837 | 1.5 | 0.72(0.53-0.97) | 0.03 | 0.80(0.58-1.11) | 0.19 | |
| rs10274587 | 5.2 | 0.72(0.53-0.97) | 0.03 | 0.80(0.58-1.11) | 0.19 | |
| rs12720066 | 4.0 | 0.97 (0.65 -1.43) | 0.87 | 0.77 (0.49-1.21) | 0.26 | |
| rs1922242 | 0.8 | 1.17 (0.99-1.37) | 0.06 | 1.10 (0.92 -1.32) | 0.29 | |
| rs2091766 | 4.6 | 1.10 (0.94 -1.29) | 0.24 | 1.04 (0.87 -1.24) | 0.67 | |
| rs2235033 | 0.3 | 0.86(0.73-1.02) | 0.09 | 0.87 (0.72-1.05) | 0.15 | |
| rs2032588 | 0.2 | 0.67(0.47-0.97) | 0.03 | 0.63 (0.42 -0.95) | 0.03 | |
| rs1128503 | 10.9 | 1.01(0.85-1.19) | 0.95 | 1.04 (0.86 -1.25) | 0.70 | |
| rs2235023 | 1.2 | 0.58 (0.40 -0.82) | 0.003 | 0.53(0.36-0.79) | 0.002 | |
| rs13237132 | 9.7 | 1.21 (1.03 -1.44) | 0.02 | 1.16(0.97-1.40) | 0.11 | |
| rs12334183 | 1.2 | 0.65 (0.51 - 0.83) | 0.0005 | 0.67(0.52-0.88) | 0.004 | |
| rs10264990 | 3.0 | 1.24 (1.05 -1.47) | 0.01 | 1.20 (1.00 -1.44) | 0.05 | |
| rs1989830 | 5.3 | 0.82 (0.68 -0.98) | 0.03 | 0.82 (0.67 -1.00) | 0.05 | |
| rs12O2172 | 2.0 | 0.82 (0.68 -0.98) | 0.03 | 0.82 (0.67 -1.00) | 0.05 | |
| rs17327442 | 0.9 | 1.12 (0.89 -1.40) | 0.33 | 1.11 (0.87 -1.42) | 0.42 | |
| rs1202184 | 1.2 | 1.12 (0.95 -1.32) | 0.19 | 1.12 (0.94 -1.35) | 0.21 | |
| rs1211152 | 1.7 | 0.94(0.71-1.25) | 0.68 | 0.91(0.65-1.26) | 0.56 | |
| rs17327624 | 2.7 | 1.10 (0.89 -1.34) | 0.37 | 1.08 (0.87 -1.35) | 0.49 | |
| rs13229143 | 0.9 | 0.83(0.70-0.99) | 0.03 | 0.85(0.71-1.03) | 0.09 | |
| rs12535512 | 0.6 | 1.03 (0.87 -1.22) | 0.75 | 1.02 (0.85 -1.23) | 0.84 | |
| rs3789243 | 8.6 | 1.11 (0.94 -1.31) | 0.23 | 1.09(0.91-1.30) | 0.36 | |
| rs2214102 | 4.1 | 0.85(0.64-1.13) | 0.27 | 0.83(0.61-1.14) | 0.25 | |
| rs4728709 | 0.4 | 1.22(0.92-1.62) | 0.18 | 1.17 (0.86-1.60) | 0.31 | |
| rs4148732 | 10.9 | 1.28 (1.01 -1.60) | 0.04 | 1.31(1.02-1.69) | 0.03 | |
| rs13233308 | 25.5 | 0.94(0.79-1.10) | 0.44 | 0.93(0.78-1.11) | 0.42 | |
| rs2157926 | 5.1 | 1.22(0.92-1.62) | 0.18 | 1.17 (0.86-1.60) | 0.31 | |
| rs10246878 | - | 0.99(0.81-1.21) | 0.89 | 1.00 (0.80 -1.25) | 0.99 | |
| High-Grade Serous (N=262) | High-Grade Serous on Platinum (N=234) | |||||
| Gene | rsid | kb to next | HR (95% CI) | p | HR (95% CI) | p |
| ERCC2 | rs10853773 | 2.4 | 1.16 (0.93 - 1.44) | 0.20 | 1.16 (0.92 - 1.46) | 0.22 |
| rs13181 | 1.2 | 1.09 (0.90 - 1.32) | 0.38 | 1.05 (0.86 - 1.29) | 0.63 | |
| rs1799787 | 0.3 | 1.21 (0.97 - 1.51) | 0.09 | 1.19 (0.94 - 1.50) | 0.15 | |
| rs238417 | 0.6 | 0.74(0.59-0.92) | 0.006 | 0.75(0.60-0.94) | 0.01 | |
| rs238416 | 0.2 | 0.79(0.63-0.98) | 0.04 | 0.83 (0.66 -1.04) | 0.10 | |
| rs238415 | 5.2 | 0.75(0.61-0.93) | 0.01 | 0.76(0.60-0.95) | 0.02 | |
| rs50872 | 0.1 | 1.20 (0.94 -1.52) | 0.14 | 1.10 (0.85 -1.42) | 0.49 | |
| rs50871 | 9.1 | 0.95(0.79-1.14) | 0.57 | 0.92(0.76-1.13) | 0.44 | |
| rs1618536 | 2.3 | 0.89(0.73-1.08) | 0.24 | 0.92(0.75-1.14) | 0.45 | |
| rs3810366 | 3.0 | 1.04(0.85-1.27) | 0.72 | 0.99 (0.80 -1.23) | 0.94 | |
| rs11878644 | - | 1.09 (0.89 -1.33) | 0.43 | 1.08 (0.86 -1.35) | 0.50 | |
Bold indicates p<=0.05
Figure 1.
Linkage disequilibrium plot of ABCB1. Haploview 4.2 based on 445 cases; r2=0=white and r2=1=black; numbers represent r2 * 100; genome build 36.3.
Table 4.
Gene-level associations
| All Cases (N=445) | High-Grade Serous (N=262) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCA | Haplotype Analysis | PCA | Haplotype Analysis | |||||||
| Gene | Chr | N SNPs | N PC | p-value | N Haplotypes | p-value | N PC | p-value | N Haplotypes | p-value |
| GSTM1 | 1 | 1 | 1 | 0.65 | na | na | 1 | 0.73 | na | na |
| XPC | 3 | 11 | 5 | 0.52 | 7 | 0.63 | 5 | 0.92 | 7 | 0.80 |
| ABCB1 | 7 | 33 | 9 | 0.003 | 3 | 0.46 | 9 | 0.36 | 3 | 0.23 |
| CYP3A4 | 7 | 5 | 3 | 0.28 | 3 | 0.62 | 3 | 0.57 | 3 | 0.69 |
| CYP2C8 | 10 | 14 | 5 | 0.09 | 6 | 0.04 | 5 | 0.43 | 6 | 0.41 |
| XRCC1 | 19 | 16 | 4 | 0.37 | 6 | 0.50 | 4 | 0.44 | 5 | 0.54 |
| ERCC2 | 19 | 11 | 5 | 0.44 | 9 | 0.59 | 5 | 0.11 | 8 | 0.05 |
| ERCC1 | 19 | 3 | 2 | 0.37 | 3 | 0.30 | 2 | 0.12 | 3 | 0.07 |
Chr, chromosome; N cases, number of cases with no missing genotypes; N PC, number of principal components into which SNPs collapsed; bold indicates p < 0.05; sorted by gene set, then by chromosomal position.
At ERCC2, a greater number of SNPs were associated at p<0.01 in high-grade serous cases (Table 3) than in analysis of all cases, suggesting heterogeneity across sub-types with risk estimates were farther from the null in the high-grade serous group. Among cases with high-grade serous disease, minor alleles at a cluster of three SNPs with r2≥0.74 were associated with outcome (e.g., rs238417 HR 0.74, 95% Cl 0.59-0.92, p=0.006). With adjustment for clinical covariates, associations remained, suggesting that genetic variation was an independent prognostic factor. Analysis of eight common ERCC2 haplotypes among patients with high grade serous disease was not statistically significant at p<0.01, but was of borderline statistical significance (p=0.05, Table 4). Among 234 high-grade serous cases treated with cisplatin or carboplatin (89%), results were similar to those in the overall group (Table 3). Thus, variation in this DNA repair gene appears related to outcome of high-grade serous disease, and this may be due to differential response to platinum-induced DNA damage.
No associations with outcome were observed for GSTM1, CYP2C8, CYP3A4, ERCC1, XPC, or XRCC1 (Table 4 and additional information will be available on request from the corresponding author).
Discussion
In a clinic-based series of invasive epithelial ovarian cancer cases, we report associations between time to recurrence or death and several SNPs in ABCB1 and ERCC2, and we detected no association with variation in GSTM1, CYP2C8, CYP3A4, ERCC1, XPC, or XRCC1. We found that adjustment for clinical factors resulted in only moderately-attenuated risk estimates at ABCB1 and ERCC2, suggesting that although SNPs may correlate with clinical features they were also associated with outcome, above and beyond these clinical features. We also found that ERCC2 was primarily associated with outcome among high-grade serous cases. Analysis of patients treated with taxanes or platinum chemotherapies (the vast majority of cases) showed consistent results. Although the current sample size did not allow for a detailed assessment of the pharmacogenetic effects (i.e., comparison of SNP associations in treated v. untreated groups using interaction models), results in treated subgroups, despite reduced sample size, are consistent with our hypothesis that variation in drug metabolism influences outcome.
Strengths of the current report include comprehensive coverage of each gene (tagging all SNPs within 5 kb with MAF≥0.05 at r2≥0.9), relatively large sample size, pathologically-confirmed diagnoses, rapid enrollment, homogenous yet representative patient population, robust genotyping, and analysis at both the SNP-level and gene-level. Limitations of this work are lack of analysis of toxicities and specific chemo-response variables (e.g., classification of patients into complete responders, partial responders, and non-responders); in addition, we did not compare SNP effects across treatment groups due to small numbers of patients not on chemo-therapeutics. Prior studies of drug-metabolizing genes in the ovarian cancer clinical trial setting (i.e., GOG and SCO-TROC1) had excellent clinical follow-up data, but were limited to assessment of only a handful of SNPs within each gene [9, 17]. Thus, true associations may have been overlooked, as the current report sought to capture all known common variation within each gene, and the previously-studied SNPs were not correlated with those of interest in the current report.
Specifically, the two ABCB1 SNPs which we found associated with outcome (rs2235023 and rs12334183) were not included in prior work. In a study of 309 Australian patients treated with paclitaxel and carboplatin geno-typed at ABCB1 rs2032582 (2677OT), rs1045642 (3435C>T), and rs1128503 (1236C>T), women with minor alleles at rs2032582 with minimal residual disease were less likely to relapse following paclitaxel/ carboplatin chemotherapy; however, analysis of SCOTROC1 cases with minimal residual disease were not consistent[4], and data from GOG were also null[5]. We studied two SNPs in LD (r2>0.9) with rs2032582 (rs1128503 and rs 12202184), and results were null in the Mayo Clinic patients. It is possible that the effect of rs2032582 appears only among a subset of women with minimal residual disease. A Swedish report found ABCB1 rs229109 (G1199T/A) to be associated with poorer outcome [32]; unfortunately, because the expected MAF of this SNP was <0.05, this SNP was not tagged in our analysis. Only one prior analyses of ERCC2 has been conducted, and this only examined one polymorphism (rs13181, K251Q) finding no association with ovarian cancer outcome [9]. While this SNP was also null in our analysis of Mayo Clinic cases; other ERCC2 SNPs were significant at p<0.01. Thus, our positive findings in ABCB1 and ERCC2 represent novel associations, previously unassessed in other study populations, and results underscore the importance of maximizing coverage of SNPs within candidate genes.
At ERCC1, we found a non-significant trend of poorer outcome (HR 1.11, 95% Cl 0.92-1.34) at rs32129386 (8092C>A), a SNP which was associated with earlier progression in GOG172, a trial of 429 women treated with taxol and cis-platinum after optimal surgery for stage III disease [17] and has been associated with poorer outcome in a Korean study of 118 patients [18]. Despite non-significant results in SCOTROC1 [9], combined or meta-analysis may yield statistically-significant results across all these and the Mayo Clinic population. At another ERCC1 SNP rs11615 (N118N), among 103 Italian patients who had surgery for advanced ovarian cancer and received platinum chemotherapy with or without taxol, patients with minor alleles had higher risk of progression and death when treated with platinum without taxol [33]. Unfortunately, we were not able to assess rs11615 due to failed genotyping, and we note that SCO-TROC1 found this to be null [9]. Other null findings in our analyses of Mayo Clinic patients are consistent with prior reports [9, 18].
In summary, we have analyzed the largest collection of drug-metabolizing polymorphisms and ovarian cancer outcome to date, and we have included the second largest collection of patients yet reported. While the genes studied were not novel, the ABCB1 and ERCC2 SNPs found to be of most interest (rs12334183 and rs238417) have not been assessed in other ovarian cancer studies; thus, of all the SNPs we assessed, these warrant investigation in other clinical populations. Prior analyses of other case collections at only a handful of polymorphisms in these drug-metabolizing genes are not definitive at the gene level. Comprehensive selection of polymorphisms chosen to be maximally informative of the underlying genetic variation, combined with gene-level statistical analysis, is critical to the elucidation of inherited factors related to outcome following ovarian cancer.
Acknowledgments
Research support was provided by NCI grants R01-CA122443 and P50-CA136393, the Fred C. and Katherine B. Andersen Foundation, and the Mayo Foundation. We thank Karin Goodman for recruitment and abstraction and Michele M. Schmidt for data management.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47:159–166. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 3.McGuire V, Jesser CA, Whittemore AS. Survival among U.S. women with invasive epithelial ovarian cancer. Gynecol Oncol. 2002;84:399–403. doi: 10.1006/gyno.2001.6536. [DOI] [PubMed] [Google Scholar]
- 4.Johnatty SE, Beesley J, Paul J, Fereday S, Spurdle AB, Webb PM, Byth K, Marsh S, McLeod H, Harnett PR, Brown R, DeFazio A, Chenevix-Trench G. ABCB1 (MDR 1) polymorphisms and progression-free survival among women with ovarian cancer following paclitaxel/carboplatin chemotherapy. Clin Cancer Res. 2008;14:5594–5601. doi: 10.1158/1078-0432.CCR-08-0606. [DOI] [PubMed] [Google Scholar]
- 5.Darcy KM, Tian C, Ambrosone CB, Krivak TC, Armstrong D, Bookman MA, Davis W, Zhao H, Moysich K, DeLoia JA. A Gynecologic Oncology Group study of associations between polymorphisms in ABC transporter genes (ABCB1, ABCC2 and ABCG2) and outcome in advanced stage epithelial ovarian cancer treated with platinum and taxane chemotherapy. J Clin Oncol. 2009;27:s293. [Google Scholar]
- 6.Harris JW, Rahman A, Kim BR, Guengerich FP, Collins JM. Metabolism of taxol by human hepatic microsomes and liver slices: participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res. 1994;54:4026–4035. [PubMed] [Google Scholar]
- 7.Rahman A, Korzekwa KR, Grogan J, Gonzalez FJ, Harris JW. Selective biotransformation of taxol to 6 alpha-hydroxytaxol by human cytochrome P450 2C8. Cancer Res. 1994;54:5543–5546. [PubMed] [Google Scholar]
- 8.Dai D, Zeldin DC, Blaisdell JA, Chanas B, Coulter SJ, Ghanayem BI, Goldstein JA. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics. 2001;11:597–607. doi: 10.1097/00008571-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Marsh S, Paul J, King CR, Gifford G, McLeod HL, Brown R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol. 2007;25:4528–4535. doi: 10.1200/JCO.2006.10.4752. [DOI] [PubMed] [Google Scholar]
- 10.Goode EL, Dunning AM, Kuschel B, Healey CS, Day NE, Ponder BA, Easton DF, Pharoah PP. Effect of germ-line genetic variation on breast cancer survival in a population-based study. Cancer Res. 2002;62:3052–3057. [PubMed] [Google Scholar]
- 11.Quintela-Fandino M, Hitt R, Medina PP, Gamarra S, Manso L, Cortes-Funes H, Sanchez-Cespedes M. DNA-repair gene polymorphisms predict favorable clinical outcome among patients with advanced squamous cell carcinoma of the head and neck treated with cisplatin-based induction chemotherapy. J Clin Oncol. 2006;24:4333–4339. doi: 10.1200/JCO.2006.05.8768. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Gurubhagavatula S, Liu G, Park S, Neuberg DS, Wain JC, Lynch TJ, Su L, Christiani DC. Excision repair crosscomplementation group 1 polymorphism predicts overall survival in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Clin Cancer Res. 2004;10:4939–4943. doi: 10.1158/1078-0432.CCR-04-0247. [DOI] [PubMed] [Google Scholar]
- 13.Park DJ, Stoehlmacher J, Zhang W, Tsao-Wei DD, Groshen S, Lenz HJ. A Xeroderma pigmentosum group D gene polymorphism predicts clinical outcome to platinum-based chemotherapy in patients with advanced colorectal cancer. Cancer Res. 2001;61:8654–8658. [PubMed] [Google Scholar]
- 14.Yuan P, Miao XP, Zhang XM, Wang ZH, Tan W, Sun Y, Xu BH, Lin DX. [Polymorphisms in nucleotide excision repair genes XPC and XPD and clinical responses to platinum-based chemotherapy in advanced non-small cell lung cancer] Zhonghua Yi Xue Za Zhi. 2005;85:972–975. [PubMed] [Google Scholar]
- 15.Nagle CM, Chenevix-Trench G, Webb PM, Spurdle AB. Ovarian cancer survival and polymorphisms in hormone and DNA repair pathway genes. Cancer Lett. 2007;251:96–104. doi: 10.1016/j.canlet.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Hogdall E, Ramus SJ, Dicioccio RA, Hogdall C, Quaye L, McGuire V, Whittemore AS, Shah M, Greenberg D, Easton DF, Kjaer SK, Pharoah PD, Gayther SA. Effects of common germ-line genetic variation in cell cycle genes on ovarian cancer survival. Clin Cancer Res. 2008;14:1090–1095. doi: 10.1158/1078-0432.CCR-07-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krivak TC, Darcy KM, Tian C, Armstrong D, Baysal BE, Gallion H, Ambrosone CB, DeLoia JA. Relationship between ERCC1 polymorphisms, disease progression, and survival in the Gynecologic Oncology Group Phase III Trial of intraperitoneal versus intravenous cisplatin and paclitaxel for stage III epithelial ovarian cancer. J Clin Oncol. 2008;26:3598–3606. doi: 10.1200/JCO.2008.16.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Kim MK, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Genetic polymorphisms affecting clinical outcomes in epithelial ovarian cancer patients treated with taxanes and platinum compounds: a Korean populationbased study. Gynecol Oncol. 2009;113:264–269. doi: 10.1016/j.ygyno.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros R, Pereira D, Afonso N, Palmeira C, Faleiro C, Afonso-Lopes C, Freitas-Silva M, Vasconcelos A, Costa S, Osorio T, Lopes C. Platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma: glutathione Stransferase genetic polymorphisms as predictive biomarkers of disease outcome. Int J Clin Oncol. 2003;8:156–161. doi: 10.1007/s10147-003-0318-8. [DOI] [PubMed] [Google Scholar]
- 20.Sellers TA, Schildkraut JM, Pankratz VS, Vierkant RA, Fredericksen ZS, Olson JE, Cunningham JM, Taylor W, Liebow M, McPherson CP, Hartmann LC, Pal T, Adjei AA. Estrogen bioactivation, genetic polymorphisms, and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2536–2543. doi: 10.1158/1055-9965.EPI-05-0142. [DOI] [PubMed] [Google Scholar]
- 21.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 23. NIEHS SNPs. NIEHS Environmental Genome Project, University of Washington, Seattle, 358 WA (URL: http://egp.gs.washington.edu) [June 2009].
- 24.Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, Vierkant RA, Armasu SM, White KL, Keeney GL, Cliby WA, Rider DN, Kelemen LE, Jones MB, Peethambaram PP, Lancaster JM, Olson JE, Schildkraut JM, Cunningham JM, Hartmann LC. Inherited determinants of ovarian cancer survival. Clin Cancer Res. 2010;16:995–1007. doi: 10.1158/1078-0432.CCR-09-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliphant A, Barker DL, Stuelpnagel JR, Chee MS. BeadArray technology: enabling an accurate, cost-effective approach to high-throughput genotyping. Biotechniques. 2002;(Suppl):56–58. 60–51. [PubMed] [Google Scholar]
- 26.Steemers FJ, Gunderson KL. Illumina, Inc. Pharmacogenomics. 2005;6:777–782. doi: 10.2217/14622416.6.7.777. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life tables (with discussion) Journal of Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 28.Freidlin B, Zheng G, Li Z, Gastwirth JL. Trend tests for case-control studies of genetic markers: power, sample size and robustness. Hum Hered. 2002;53:146–152. doi: 10.1159/000064976. [DOI] [PubMed] [Google Scholar]
- 29.Gauderman WJ, Murcray C, Gilliland F, Conti DV. Testing association between disease and multiple SNPs in a candidate gene. Genet Epidemiol. 2007;31:383–395. doi: 10.1002/gepi.20219. [DOI] [PubMed] [Google Scholar]
- 30.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett JC, Fry B, Mailer J, Daly MJ. Hap-loview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 32.Green H, Soderkvist P, Rosenberg P, Horvath G, Peterson C. ABCB1 G1199A polymorphism and ovarian cancer response to paclitaxel. J Pharm Sci. 2008;97:2045–2048. doi: 10.1002/jps.21169. [DOI] [PubMed] [Google Scholar]
- 33.Smith S, Su D, Rigault de la Longrais IA, Schwartz P, Puopolo M, Rutherford TJ, Mor G, Yu H, Katsaros D. ERCC1 genotype and phenotype in epithelial ovarian cancer identify patients likely to benefit from paclitaxel treatment in addition to platinum-based therapy. J Clin Oncol. 2007;25:5172–5179. doi: 10.1200/JCO.2007.11.8547. [DOI] [PubMed] [Google Scholar]