Abstract
Activation in frontopolar cortex (FPC; BA 10) has been associated both with attending to mental states and with integrating multiple mental relations. However, few previous studies have manipulated both of these cognitive processes, precluding a clear functional distinction among regions within FPC. To address this issue, we developed an fMRI task that combined mentalizing and relational integration processes. Participants saw blocks of single words and performed one of three judgments: how pleasant or unpleasant they found each word (Self condition), how a specific friend would evaluate the pleasantness of the word (Other condition), or the difference between their own pleasantness judgment and that of their friend (Relational condition). We found that medial FPC was modulated by Other relative to Self judgments, consistent with a role in mentalizing. Lateral FPC was significantly activated during Relational compared to Self judgements, suggesting that this region is particularly involved in relational integration. The results point to a strong functional dissociation between medial and lateral FPC. In addition, the data demonstrate a role for lateral FPC in the social domain, provided that the task requires the integration of one’s preferences with those of others.
Keywords: Frontopolar cortex, mentalizing, relational integration, social cognition, fMRI
INTRODUCTION
The frontopolar cortex (FPC) has become an important target of recent cognitive neuroscience research. Often defined as Brodmann’s area 10, and also known as rostral or anterior prefrontal cortex (Ramnani and Owen, 2004; Gilbert et al., 2006b; Burgess et al., 2007a; Smith et al., 2007), the FPC comprises the anterior extent of the frontal lobe. As FPC is greatly expanded in the human compared to nonhuman primate brain and matures relatively late in human development, it may play a critical role in higher order cognitive operations that are central to human behavior.
Recent functional imaging research has related FPC to some of the most complex aspects of cognitive function, with medial regions implicated in social cognition, and lateral regions implicated in a variety of reasoning tasks. Specifically, social cognitive accounts suggest a role for the medial FPC in tasks that involve attending to one’s own feelings and thoughts (Gusnard et al., 2001; Zysset et al., 2003; Gusnard, 2005; Rameson et al., 2010). Similar results have been reported in studies that require participants to adopt a first- vs third-person perspective (Ruby and Decety, 2001, 2004; Vogeley et al., 2004; Aichhorn et al., 2006; David et al., 2006, 2008; D’Argembeau et al., 2007), as well as in memory studies in which items are encoded in terms of their personal relevance (Craik et al., 1999; Kelley et al., 2002; Macrae et al., 2004). Additionally, medial FPC is also recruited during tasks that require attending to the mental states of others (Decety and Sommerville, 2003; Frith and Frith, 2003; Ochsner et al., 2004; Amodio and Frith, 2006). In particular, inferring others’ beliefs, intentions and emotions produces differential activation depending on the degree of perceived similarity between self and other. Mentalizing about a similar other has been shown to engage a region of the ventral medial prefrontal cortex, previously linked to self-referential thought, while mentalizing about a dissimilar other has been associated with a more dorsal region of the medial prefrontal cortex (Mitchell et al., 2005, 2006; Ames et al., 2008; Jenkins et al., 2008). Collectively, these studies suggest a core functional role for medial FPC in the shared representation of self and others’ mental states.
In contrast to this social cognitive view, recent functional accounts of lateral FPC focus on the computations critical for efficient information processing and complex reasoning. Included in these accounts have been a number of postulated processes: an attentional bias toward internally generated information (Shallice and Burgess, 1996; Christoff and Gabrieli, 2000; Simons et al., 2006; Burgess et al., 2007a, b), the implementation of hierarchically structured task goals (Koechlin et al., 2000, 2003; Braver and Bongiolatti, 2002; Badre and Wagner, 2004; Botvinick et al., 2008; Paxton et al., 2008; Badre and D’Esposito, 2009), and the comparison and integration of abstract relationships (Waltz et al., 1999; Christoff et al., 2001, 2003; Kroger et al., 2002; Bunge et al., 2005; Reynolds et al., 2006; DiPisapia et al., 2007; Smith et al., 2007; Wendelken et al., 2008). Earlier studies used adapted versions of the Raven’s Progressive Matrices, where multiple dimensions have to be compared to determine the relationships between objects (Christoff et al., 2001; Kroger et al., 2002; Ramnani and Owen, 2004). Consistently across studies, activation in lateral FPC increased with the number of relations that were simultaneously considered. Furthermore, lateral FPC is more active when participants evaluate analogies (e.g. is shoe to foot as glove is to hand?) than when they evaluate semantic relations (e.g. is rain related to drought?), suggesting that this region is particularly involved in integrating across multiple retrieved relations (Bunge et al., 2005; Green et al., 2006). As noted in several articles of lateral FPC function, the variety of paradigms that evoke FPC activation have posed challenges for any one functional account (e.g. Ramnani and Owen, 2004). Moreover, some accounts may share cognitive processes. For example, relating the outcomes of two or more cognitive operations in pursuit of a higher goal is likely to require both considerable stimuli-independent attending and the evaluation of self-generated information (Bunge et al., 2009).
One clear trend is the implication of medial FPC in social cognitive tasks linked to processing of one’s own mental state or of the mental states of others. In contrast, lateral FPC activation is often observed in complex relational reasoning tasks requiring the comparison of internally generated or discovered relationships. Yet, social cognition can be relational. A core component of social cognition is the ability to consider one’s own intentions, actions or preferences in light of social norms or other normative standards (Cialdini and Goldstein, 2004; Amodio and Frith, 2006; Klucharev et al., 2009; Krueger et al., 2009). So, to the extent that lateral FPC is critical for the comparative processing of abstract relationships, it should play a role in specific aspects of social cognition, namely considering one’s own judgments or preferences in light of another’s. Recent reports support this conjecture (David et al., 2006; Mitchell et al., 2009). In a task where participants had to make inferences about unfamiliar others, Mitchell and colleagues found greater lateral PFC when participants applied gender stereotypes than when participants avoided stereotype use (Mitchell et al., 2009). Thus, it may be that lateral FPC is implicated in social cognitive tasks, as long as they require relating one’s preferences to social standards and beliefs. Moreover, in a visuospatial perspective-taking task, David et al. (2006) found increased lateral FPC for third- compared to first-person perspective. Importantly, while both perspectives require establishing a subject-to-object relation, the former necessitates an additional translocation of the viewpoint (Vogeley et al., 2004). Therefore, one may postulate that a third-person perspective task requires processing and integration of multiple relations, whereas the first-person perspective task only requires processing of a single relation.
To address the possibility that lateral FPC is implicated in relational aspects of social cognition, we developed a task that combined the two classes of cognitive functions previously associated with FPC recruitment: mentalizing and relational integration. Critically, we used the same stimulus probes to trigger both sorts of processing: some conditions required making single decisions about self and others, while another condition required relating two judgments and integrating them in a response. In contrast to earlier studies, our stimuli and tasks were all social in nature, allowing us to investigate whether lateral FPC regions may be implicated in social cognition. In addition, our task keeps the need to generate and orient attention toward internal information constant, since in all conditions information could not be perceived from the external environment but needed to be generated internally based on prior knowledge about self and other. Hence, any activation differences in FPC regions between conditions should reflect relational operations rather than self-directed processing in the social domain.
We also examined the potential functional dissociation between medial and lateral regions. Recent meta-analyses of imaging studies have reported distinct activation peaks within BA 10 along the medial–lateral axis (Gilbert et al., 2006a, b; Burgess et al., 2007b). As mentioned above, mentalizing and self-reflection tasks tend to involve significantly more medial regions relative to studies of multitasking and relational integration (Gilbert et al., 2006a, b; Burgess et al., 2007b). Turner et al. (2008) provided some compelling evidence for the medial–lateral dissociation within the same group of participants. In a source memory study, they showed that medial FPC was engaged during recollection of self-generated information, whereas lateral FPC played a more general role in source recollection. Such within-participant dissociations are rare, and thus convincing evidence for functional segregation requires further within-participants imaging data that holds stimulus material constant (Gilbert et al., 2007), as in the present study. Furthermore, we investigated whether the tendency to recruit medial and lateral FPC regions was predicted by relevant individual-difference measures. Previous work showed that mentalizing skills are related to the ability to empathize with others and demonstrate altruistic concern (Seitz et al., 2006; Lamm et al., 2007; Hooker et al., 2008). Here, we examined whether differential activation across participants in specific FPC regions during mentalizing vs relational integration predicted self-reported altruism and empathic concern.
In summary, the current experiment contrasted judgments about the self, another and the self in relation to another. Because the stimuli probed for these judgments are fixed, and because all three tasks required internally generated content, dissociations across the task likely represent differences in the computations underlying the judgments, not simple differences in stimuli or attentional focus. Furthermore, the design allowed us to test whether lateral FPC activation could be observed in an inherently social judgment and to investigate whether FPC activation varied with individual differences in personality characteristics linked to social behaviors.
METHODS
Participants
Twenty-five healthy, neurologically normal volunteers (15 female; age range: 18–25 years; mean age: 21 years) were included in the study. Informed consent was obtained in a manner approved by the Institutional Review Board of Duke University Medical Center. Behavioral data from one participant were not recorded correctly because of a technical problem with the response box. This participant’s data were excluded from the behavioral analysis (n = 24), but the fMRI data were included in the determination of the activation maps (n = 25). Psychometric data from two participants were lost due to a computer error, and results from another two participants were >2 s.d. above the mean. Therefore, these four participants were excluded before analyses of correlations with personality variables (n = 21).
Materials and procedure
At the beginning of the experimental session, before entering the fMRI scanner, participants were asked to select and think about a friend whom they knew very well but whose opinions, attitudes and tastes were very different from their own. During the fMRI session, participants saw single words and were asked one of four types of questions, depending on the task condition (Figure 1). In the Self condition, participants had to decide how pleasant or unpleasant they found each word. In the Other condition, participants were asked how pleasant or unpleasant they thought their friend would find each word. In the Relational condition, they had to decide how much more pleasant or unpleasant than their friend they found each word (Figure 1A). Participants were instructed that questions in the Other and Relational conditions referred to the friend that they had previously selected. Finally, the Control blocks required counting the number of vowels in each word.
Fig. 1.
Schematic diagram of the experimental paradigm. (A) An event from each experimental block. After an initial warning screen, participants saw single words and were asked three types of questions corresponding to the Self, Other and Relational tasks. Participants responded using an 8-point scale. (B) Each run comprised five experimental blocks, randomized among the three tasks (Self, Other and Relational), lasting 60 s each. Experimental blocks were interspersed by Control blocks with a 30 s duration. Cont. = Control.
Participants responded using an eight-button input device with buttons distributed over two boxes (one for each hand) so that the numbers 1 through 8 were each selected by one finger in order from left to right. For Self and Other conditions, a response of 1 denoted ‘very unpleasant’ and 8 denoted ‘very pleasant’. In the Relational condition, the endpoints indicated that a given probe was rated as ‘much less pleasant’ and ‘much more pleasant’, respectively. That is, for the Relational condition the chosen friend constituted the scale’s midpoint or anchor. All stimuli were pseudo-randomly selected from a pool of 1200 common words which were, on average, 7.09 letters and 2.34 syllables long, with a Kucera–Francis corpus frequency of 8.85.
The task was divided into four runs. After an initial warning screen, a white fixation cross was presented on a black background for 8 s. Participants were then presented with five blocks, each consisting of a 60-s subblock from one of the three experimental conditions (Self, Other or Relational), followed by a 30-s subblock of Control questions (Figure 1B). The order of conditions was pseudo-randomized within each set of three blocks, including across run breaks. Presentation of probe words was self-paced; participants could take as much time as necessary to answer each question. After responding, their response was briefly displayed on the screen, then a fixation cross was displayed briefly before another question of the same type was presented. On average, participants viewed 230 (s.e. = 3.2) words from the experimental conditions and 124 (s.e. = 2.9) words from the control conditions.
Following the task in the scanner, participants completed additional psychometric tests that assessed individual differences potentially linked to social cognition, mentalizing and relational thought. Participants completed the Personal Altruism Level (PAL) scale (Tankersley et al., 2007) and the Self-Report Altruism Scale (SRAS; Rushton et al., 1981), which contain questions about how often the participant engages in a range of altruistic behaviors (e.g. giving directions to a stranger; donating to charity). Participants also completed two other psychometric surveys. The Interpersonal Reactivity Index (IRI; Davis, 1980) provides measures of four components of empathy: empathic concern, fantasy, personal distress and perspective taking. The Neuroticism–Extroversion–Openess (Costa and McCrae, 1992) inventory assesses the basic personality traits of agreeableness, conscientiousness, extraversion, openness and neuroticism.
fMRI acquisition and analysis
Scanning was performed on a 3T GE scanner with a multichannel (eight coil) parallel imaging system. Functional data were acquired using a gradient echo sequence (TR = 2000 ms, TE = 27 ms, 32 slices parallel to the AC–PC plane, with voxel size of 3.75 × 3.75 × 3.8 mm). High-resolution 3D full-brain anatomical images were acquired to aid in normalization and coregistration of individual participants’ data. Before functional data collection, four dummy volumes were discarded to allow for T1 equilibration. fMRI data were processed and analyzed using FSL 4.0 (FMRIB Software Library; Smith et al., 2004). Functional images were corrected for head motion and time of acquisition within a TR and were normalized into a standard stereotaxic space (Montreal Neurological Institute) for interparticipant comparison using the FMRIB’s Linear Image Registration Tool component of FSL. A smoothing filter of width 8 mm was applied after normalization.
Functional MRI data were processed with FEAT 5.92 (FMRI Expert Analysis Tool) in FSL using an event-related analysis, which often provides a more accurate model of the haemodynamic responses than an epoch-related analysis, even in blocked design fMRI (Mechelli et al., 2003). Moreover, since responses were self-paced, introducing some jittering between onsets of trials, an event-related analysis allowed us to time lock the fMRI images to the onset of the stimulus presentation. The model was constructed with a regressor for each condition (Self, Other, Relational and Control). Each regressor was modelled for the full response interval for each participant, time locked to the onset of the trial presentation and convolved with a canonical double-gamma haemodynamic response function. Response times (RTs) were included in the model, as another regressor, to control for activation differences that may emerge as a result of differences in RTs between conditions. The estimated regression parameters were combined across runs using a fixed-effects analysis and were combined across participants using a mixed-effects analysis (FLAME-1). Clusters of activation exceeding threshold of z >2.3 are reported, yielding a family-wise corrected P-value of less than 0.001 for the entire brain volume based on Gaussian Random Field Theory (Worsley et al., 1992). The Feat query component of FSL extracted percent signal change for significant voxels within regions of interest (ROIs).
RESULTS
Behavioral data
Since participants’ responses were self-paced, the number of items presented varied across conditions. Specifically, participants responded, on average, to 124 (s.e. = 2.9) trials in the Control task, 86 (s.e. = 2.7) trials in the Self task, 78 (s.e. = 2.9) trials in the Other task and 68 (s.e. = 3.2) trials in the Relational task. These differences were accompanied by a significant variation in RTs across conditions (Control = 2650 ms, Self = 2690 ms, Other = 3270 ms, Relational = 4010 ms; P < 0.001 in all cases, except between Control and Self where P > 0.1). Consistent with our hypotheses, these data suggested that task complexity demands increased from Self to Other to Relational trials. Therefore, we included RTs as a parametric covariate in the fMRI model to rule out concerns that activation differences across conditions could be due to longer time on task.
Functional imaging data
We first investigated the neural regions associated with the processing of mental states by comparing each experimental condition (Self, Other and Relational) with the Control condition (vowel counting). All three experimental tasks evoked activation in a network of regions including bilateral medial FPC (BA 10), L ventrolateral (BA 47, 45, 44) and dorsolateral prefrontal cortex (BA 9), L premotor cortex (BA 8, 6), L superior temporal pole (BA 38) and L middle and inferior temporal gyrus (BA 21). Activation was also found bilaterally in anterior cingulate gyrus (BA 32, 24), and in L lingual gyrus (BA 18) and cerebellum. Consistent with previous studies, these results provide evidence for shared processes underlying inferences about the mental characteristics of others and processing information about the self (Decety and Sommerville, 2003; Ochsner et al., 2004; Seger et al., 2004; Mitchell et al., 2005).
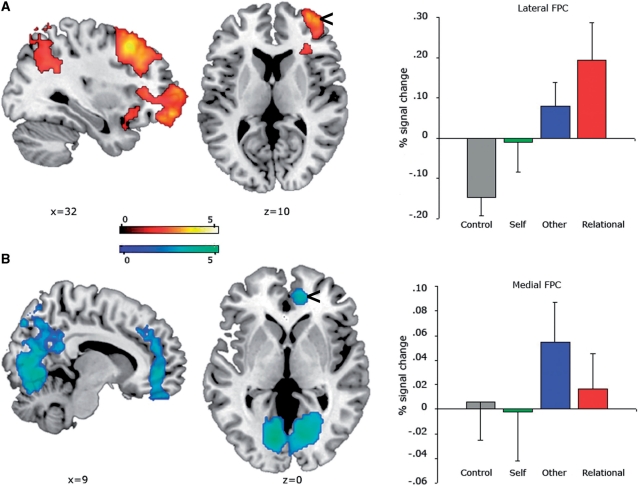
To explore the differential effects of relational integration and mentalizing, we examined the Relational > Self, Other > Self and Relational > Other contrasts (Figure 2, Table 1). If lateral FPC regions mediate relational reasoning, but are not specific to internally directed processes, then the Relational (but not the Other) task should evoke lateral FPC activation. Consistent with this hypothesis, we found increased activation in R lateral FPC (BA 10) for Relational compared to Self judgments (Figure 2A). In addition to this region, several other activation clusters were observed: bilateral ventrolateral (BA 45, 47) and dorsolateral prefrontal cortex (BA 9, 46), bilateral middle temporal gyrus (BA 21, 39), bilateral parietal lobule (BA 40), anterior and posterior cingulate gyrus (BA 32, 23) and R insula (BA 48). No regions exhibited significant activation in the Self > Relational contrast, at the standard statistical threshold. However, decisions about another person relative to judgments about oneself (Other > Self) produced significant activation in medial FPC (BA 10, Figure 2B). Additional clusters were observed predominantly in L ventrolateral (BA 44) and L dorsolateral prefrontal cortex (BA 9, 46), bilateral middle temporal and occipital (BA 39), lingual (BA 18) and anterior cingulate gyrus (BA 32). The opposite contrast of Self > Other did not show any significant activation. Finally, the contrast of Relational > Other showed a significant bilateral activation in the inferior parietal lobule (BA 39/40) but no significant differences in FPC or other frontal regions. Since the two conditions share many cognitive and emotional processes, the lack of FPC differences is not surprising.
Fig. 2.
Differential effects of relational integration and mentalizing. (A) Regions demonstrating significant increases of activation in Relational > Self decisions. Plots show the effect sizes for each condition in the lateral FPC (8-mm sphere around the peak activation at [42 56 6]). (B) Regions demonstrating significant increases of activation in the Other > Self decisions. Plots show the effect sizes for each condition in the medial FPC (8-mm sphere around the peak activation at [10 46 −2]). Activations are overlaid on a canonical brain and thresholded using clusters determined by z > 2.3, and a corrected cluster significance threshold of P < 0.001. Error bars indicate the standard error.
Table 1.
Regions demonstrating significant increases in response to Relational relative to Self task, and to Other relative to Self task
| Contrast | Region/cluster size | Subregion | BA | Max Z | Subregion maxima |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Relational > Self | Parietal/12 266 | L inferior parietal | 40 | 4.82 | −38 | −50 | 50 |
| 4.51 | −6 | −60 | 44 | ||||
| 4.14 | −50 | −44 | 50 | ||||
| L Precuneus | 23 | 4.51 | −6 | −60 | 44 | ||
| 7 | 4.21 | −8 | −60 | 52 | |||
| L Angular | 39 | 4.28 | −48 | −54 | 38 | ||
| Frontotemporal/10 478 | R superior frontal | 8 | 4.72 | 30 | 18 | 50 | |
| 6 | 4.7 | 28 | 14 | 44 | |||
| L dorsolat. prefrontal | 9 | 4.57 | −36 | 14 | 44 | ||
| L ventrolat. prefrontal | 45 | 4.22 | −44 | 32 | 30 | ||
| 44 | 4.17 | −36 | 20 | 34 | |||
| R lateral frontopolar | 10 | 4.18 | 42 | 56 | 6 | ||
| Other > Self | Occipital/14 380 | L lingual | 18 | 5.2 | −12 | −72 | −8 |
| 18 | 4.46 | −10 | −62 | −6 | |||
| R lingual | 18 | 4.47 | 14 | −74 | 2 | ||
| 18 | 4.45 | 18 | −64 | −8 | |||
| 18 | 4.34 | 14 | −68 | −2 | |||
| R Cerebellum | − | 4.19 | 18 | −72 | −14 | ||
| Frontal/2958 | L dorsolat. prefrontal | 46 | 3.92 | −32 | 18 | 38 | |
| L superior frontal | 6 | 3.45 | −38 | 6 | 56 | ||
| 3.44 | −34 | 4 | 54 | ||||
| L orbital frontal | 11 | 3.44 | −6 | 48 | −14 | ||
| 3.34 | −2 | 44 | −16 | ||||
| R medial frontopolar | 10 | 3.39 | 10 | 46 | −2 | ||
L = left; R = right; dorsolat = dorsolateral; ventrolat = ventrolateral.
Functional ROIs were extracted for the lateral and medial regions of the FPC activated in the contrasts of Relational > Self and Other > Self, respectively. Percent signal averages were obtained for the significant voxels within a cluster as defined by an 8 mm radius around each identified maxima in the two regions. The plots of percent signal changes for each condition against an implicit baseline showed that lateral and medial regions were sensitive to the task performed (Figure 2). We inspected the effect sizes of these peak activations to further explore the differences between relational and mentalizing tasks. A repeated measures analysis of variance (ANOVA) with factors of task condition (Control vs Self vs Other vs Relational) and neural region (medial vs lateral FPC) yielded a significant interaction across regions across all four conditions [F(3,72) = 4.15, P < 0.05]. Even though the ANOVA was performed on nonindependent ROIs extracted from the fMRI contrasts (Poldrack and Mumford, 2009), the results provide additional evidence for differential effects of task in each region. Follow-up comparisons indicated a reversed association across the Relational and Other conditions, with greater activity in the lateral region for the Relational task and greater activity in the medial region for the Other task [F(1, 24) = 6.49, P < 0.05]. This pattern cannot be explained as complementary activations and deactivations between the regions, given that the Other and Relational conditions were greater than the Self and Control for both regions. This selective dissociation indicates that the two regions are functionally distinctive (Henson, 2006), with lateral regions responding to relations and integration of information and medial regions supporting processing associated with mentalizing.
Having established that relational integration and mentalizing processes yield differential activation in lateral and medial FPC regions, we next investigated whether such differences were modulated by distinct personality characteristics by examining the correlations between the personality scales and blood oxygen level dependence (BOLD) response within the above mentioned ROIs. In the lateral FPC ROI, we directly correlated activation for Relational > Control with responses to each personality scale. A similar analysis was carried out in the medial FPC ROI between activation for Other > Control and the psychometric data. The Control task was used as the comparative condition because we assume that the vowel counting task was not systematically related to the personality constructs. Activation in the lateral FPC for Relational compared to Control judgments was negatively correlated with the Fantasy scale of the IRI (r = −0.67, P < 0.001). Conversely, the SRAS showed a significant negative correlation with medial FPC activation in the Other relative to Control task (r = −0.52, P < 0.05). Direct comparisons of the correlation coefficients in each region showed that in lateral FPC the Relational > Control signal difference was more correlated with fantasy than altruism trait, with the P-value approaching significance (P = 0.075). In contrast, activation for Other > Control in medial FPC was significantly more correlated with altruism than fantasy trait (P < 0.05). Note that scores on these two personality measures (fantasy and altruism) were not correlated across participants (r = 0.13, P > 0.1). No other psychometric measures were significantly correlated with FPC recruitment.
DISCUSSION
We sought to distinguish the cognitive functions associated with lateral and medial FPC, using a novel mentalizing task with multiple integration requirements. The Self and Other conditions involved generating a representation of oneself and another person’s preferences about common words. The Relational condition was a more sophisticated mentalizing task that required participants to compare their own judgments with their beliefs about another person’s judgments. Thus, although the stimuli were identical across conditions, only the Relational task required participants to integrate two judgments in order to produce a response.
As predicted by the relational integration hypothesis (Christoff et al., 2001; Krogger et al., 2002; Bunge et al., 2005, 2009; Wendelken et al., 2008), we found lateral FPC activation for Relational compared to Self decisions. Since both conditions required participants to evaluate internally generated information about the pleasantness of words, our results challenge accounts that postulate a specific role of this region in the maintenance of internally directed or stimuli-independent thought (Christoff et al., 2003; Gilbert et al., 2005; Burgess et al., 2007a, b; Smith et al., 2007). Conversely, our results are consistent with prior studies which have demonstrated lateral FPC involvement during the evaluation of relationships, whether visuospatial (Waltz et al., 1999; Kroger et al., 2002; Christoff et al., 2003; Smith et al., 2007; Bunge et al., 2009) or verbal and semantic relations (Braver and Bongiolatti, 2002; Bunge et al., 2005; Green et al., 2006). For example, this region has been shown to be significantly activated during 2-relational relative to 1-relational and 0-relational problems of the Raven’s Progressive Matrices (Christoff et al., 2001), as well as during the evaluation of propositional analogies (e.g. ‘is shoe to foot as glove is to hand?’; Bunge et al., 2005; Green et al., 2006). Consistently across studies, lateral FPC shows sensitivity to the manipulation and integration of multiple relations between thoughts to execute a higher order cognitive goal (Waltz et al., 1999; Christoff et al., 2001, 2003; Kroger et al., 2002; Bunge et al., 2005; DiPisapia et al., 2007; Wendelken et al., 2008). However, in contrast to these studies that have focused on high-level cognitive tasks, here we demonstrate a role for lateral FPC in the social domain, provided that the task requires considering the relationship between one’s preferences and those of a conspecific.
Our activation in the lateral FPC was very close to a region reported by David et al. (2006) in a visuospatial task that contrasted third- and first-person perspective taking. Our results support the view that this region plays an important role in processes involved in self-other distinctions. Moreover, our data extend these prior findings by showing that lateral FPC is particularly involved in comparing and integrating information about self and others. Consistent with this view, the third-person perspective task requires processing and integration of two relations (subject-to-subject and subject-to-object), whereas the first-person task merely requires identification of a single relation (subject-to-object; Vogeley et al., 2004). In another study, Mitchell et al. (2009) found that lateral FPC was more engaged when participants made stereotyped decisions about others relative to nonstereotyped judgments. The authors suggested that R frontal cortex activation reflects semantic retrieval of categorical knowledge during stereotype application. In our study, it is plausible that similar retrieval mechanisms take place during the Relational condition, as participants attempt to retrieve semantic and/or episodic information about their friend. However, a retrieval perspective would predict increased lateral FPC not only during Relational judgments, but also in the Other condition, as in both cases participants must retrieve information about a close person. Our fMRI data argues against this interpretation, as the contrast of Other relative to Self demonstrated medial but not lateral FPC recruitment. Our findings are more easily reconciled with the relational integration hypothesis of the lateral FPC. In a similar vein, one way to interpret Mitchell’s stereotype data is to note that using stereotypes may involve relational reasoning. Although stereotypes are often activated automatically upon encountering a social group member, stereotyping may also arise following deliberative processes (Mitchell et al., 2009). For instance, one must consider how an individual may relate to a specific social group and how that group relates to certain values and preferences.
Activation within the right lateral FPC increased from Self to Other to Relational judgments, mirroring the effect on RT. This pattern is consistent with previous studies showing sustained activation in lateral FPC as a function of task difficulty (Braver et al., 2003; Velanova et al., 2003) and when participants are aware of the demands of the upcoming task (Dobbins and Han, 2006a). However, two aspects of our experiment argue against the attribution of lateral FPC activation to task difficulty: we included RTs as covariates in the fMRI model, and the Self and Other conditions evinced a significant difference in RT but no difference in lateral FPC activation. Prior research has also indicated that the nature of the decision rules that participants must make (e.g. same-different decision vs two-alternative force choice) can influence lateral FPC activation (Dobbins and Han, 2006b). Similarly to other recent studies (Smith et al., 2007; Bunge et al., 2009), we used a block design that minimized the need for rule retrieval and maximized detection power.
In contrast to lateral FPC, medial FPC was particularly engaged by the Other condition. Several studies have demonstrated that inferences about another’s mental states rely on medial aspects of FPC (Ruby and Decety, 2001, 2004; Mitchell et al., 2002, 2005, 2006; Frith and Frith, 2003; Ochsner et al., 2004; Amodio and Frith, 2006; Ames et al., 2008; Jenkins et al., 2008). In a recent study, Jenkins and colleagues (Jenkins et al., 2008) found medial FPC activation during judgments about mental states of others who are perceived to be similar to oneself, but not when the other was perceived as dissimilar in terms of preferences. Note that we observed medial FPC activation in the latter case, i.e. from judgments about a friend who was deemed dissimilar. One possible reconciliation between our results and those of Jenkins and colleagues is that when adopting the other’s perspective our participants may still have considered similarities between their own and the other’s preferences. We should note that participants were instructed to think about a friend whom they knew very well. A recent meta-analysis showed that close others (such as mother, relatives and friends) activate medial FPC, even when perceived as dissimilar to the self, perhaps due to emotional attachment or perceived cognitive similarity (Van Overwalle, 2009). This meta-analysis, along with our results, point to the notion that the medial FPC effect is modulated by the closeness of the relationship.
Engagement of medial FPC during Other judgments may be related to other processes that are recruited when adopting someone else’s viewpoint, namely inferring the person’s perspective, inhibiting the tendency to attribute one’s own preferences to others and retrieving past experiences with the friend in order to assist with judgments about this person (Ruby and Decety, 2004; D’Argembeau et al., 2007). In either case, our results extend the current views about mentalizing by demonstrating that lateral FPC is recruited when integrating the results of judgments about self and others. Thus, lateral FPC may support human social interaction by interpreting behaviors according to social preferences and norms (Klucharev et al., 2009; Krueger et al., 2009).
Our observed medial vs lateral FPC dissociation fits well with research showing that the two regions are cytoarchitectonically different (Petrides and Pandya, 1999; Burman et al., 2006), and meta-analyses indicating that studies involving mentalizing tend to recruit more medial regions compared to studies on multitasking (Gilbert et al., 2006b). The medial and lateral regions also dissociated in terms of sensitivity to individual differences in altruistic and relational personality traits. Specifically, participants who reported being generally less altruistic in the SRAS demonstrated more pronounced medial FPC signal during mentalizing (i.e. Other > Control trials). In contrast, participants with lower scores in the Fantasy scale of the IRI showed greater lateral FPC activation during Relational > Control trials. This scale evaluates the tendency to imaginatively transpose oneself into fictional situations (e.g. ‘when I watch a good movie, I can very easily put myself in the place of a leading character.’), in effect comparing oneself with another agent. Direct comparisons of the correlation coefficients in each region further suggested a functional dissociation between the two regions of FPC, such that in the lateral FPC the Relational > Control signal difference was more correlated with fantasy than altruism trait, whereas activation for Other > Control in medial FPC was significantly more correlated with altruism than fantasy trait. However, these findings should be treated with caution as brain–behavior correlations often differ greatly between studies (Decety and Batson, 2009). Moreover, the precise cognitive processes underlying the psychometric scales are unknown, with some authors proposing that the fantasy subscale measures automatic emotional empathy without necessarily involving a relational component (Fukushima and Hiraki, 2009).
Our findings point to several avenues of future research on the role of lateral and medial FPC regions in social behavior. Although lateral FPC is typically associated with complex, purely cognitive tasks, our results suggest that it may also play a key role in social cognition. Appreciating the similarities and differences between one’s own preferences and those of another person is a core social skill that facilitates the adaptive control of behavior. In its absence, a range of behaviors both positive (e.g. empathetic behavior) and negative (e.g. political deception) would be impaired. Future studies may include finer grained analyses of the interactions between medial and lateral FPC, namely how these two regions may cooperate to facilitate the complex skills underlying judgments about social norms and preferences. Our study also raises the possibility that medial FPC encodes the closeness of the relationship between self and others. An important goal for future studies concerns unraveling the specific mechanisms underlying medial FPC activity, namely the role of emotional attachment generated by familiar others (Van Overwalle et al., 2009), the perceived similarity between self and other (Mitchell et al., 2006) and/or the retrieval of episodic and autobiographical memories (Maguire et al., 2001).
Thus, using a task that incorporates mentalizing and relational integration processes, we provide support for a functional dissociation along the medial–lateral axis of the FPC. Medial FPC responses were observed when participants made judgments about familiar others, whereas recruitment of an additional region in the lateral FPC is required for integrating the results of judgments about self and others.
Conflict of Interest
None declared.
Acknowledgments
This research was supported by grants NS41328 and MH70685 to S.A.H., and MH073982 to I.G.D. We thank Jason Chen for assistance in task programming and David V. Smith for helpful discussions about the neuroimaging analysis.
REFERENCES
- Aichhorn M., Perner J., Kronbichler M., Staffen W., Ladurner G. Do visual perspective tasks need theory of mind? NeuroImage. 2006;30:1059–68. doi: 10.1016/j.neuroimage.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Ames D.L., Jenkins A.C., Banaji M.R., Mitchell J.P. Taking another’s perspective increases self-referential neural processing. Psychological Science. 2008;19:642–4. doi: 10.1111/j.1467-9280.2008.02135.x. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D., D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nature Reviews Neuroscience. 2009;10:659–69. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D., Wagner A.D. Selection, integration, and conflict monitoring: Assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–87. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M. Hierarchical models of behavior and prefrontal function. Trends in Cognitive Sciences. 2008;12:201–8. doi: 10.1016/j.tics.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S., Bongiolatti S.R. The role of frontopolar prefrontal cortex in subgoal processing during working memory. NeuroImage. 2002;15:523–36. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Braver T.S., Reynolds J.R., Donaldson D.I. Neural nechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–26. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Wendelken C., Badre D., Wagner A.D. Analogical reasoning and prefrontal cortex: Evidence for separable retrieval and integration mechanisms. Cerebral Cortex. 2005;15:239–49. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Helskog E.H., Wendelken C. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. NeuroImage. 2009;15:338–42. doi: 10.1016/j.neuroimage.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P.W., Gilbert S.J., Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philosophical Transactions of the Royal Society of London B. 2007a;362:887–99. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P.W., Gilbert S.J., Dumontheil I. The gateway hypothesis of rostral PFC (area 10) function. Trends in Cognitive Science. 2007b;11:290–8. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burman K.J., Palmer S.M., Gamberini M., Rosa M.G. Cytoarchitectonic subdivisions of the dorsolateral frontal cortex of the marmoset monkey (Callithrix), and their projections to dorsal visual areas. Journal of Comparative Neurology. 2006;495:149–72. doi: 10.1002/cne.20837. [DOI] [PubMed] [Google Scholar]
- Christoff K., Gabrieli J.D.E. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–86. [Google Scholar]
- Christoff K., Prabhakaran V., Dorfman J., et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage. 2001;14:1136–49. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Christoff K., Ream J.M., Geddes L.P.T., Gabrieli J.D.E. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behavioral Neuroscience. 2003;117:1161–8. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Cialdini R.B., Goldstein N.J. Social influence: compliance and conformity. Annual Review Psychology. 2004;55:591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- Costa P.T., McCrae R.R. NEO PI-R. Professional manual. Odessa, FL: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- Craik F.M., Moroz T.M., Moscovitch M., et al. In search of the self: A positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- D’Argembeau A., Ruby P., Collette F., et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective-tasking? Journal of Cognitive Neuroscience. 2007;19:935–44. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- David N., Bewernick B.H., Cohen M.X., et al. Neural representations of self versus other: Visual-spatial perspective taking. Journal of Cognitive Neuroscience. 2006;6:898–910. doi: 10.1162/jocn.2006.18.6.898. [DOI] [PubMed] [Google Scholar]
- David N., Aumann C., Santos N.S., et al. Differential involvement of the posterior temporal cortex in mentalizing but not perspective taking. Social, Cognitive and Affective Neuroscience. 2008;3:279–89. doi: 10.1093/scan/nsn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- Decety J., Batson C.D. Empathy and morality: Integrating social and neuroscience approaches. In: Braeckman J., Verplaetse J., De Schrijver J., editors. The Moral Brain. Berlin: Springer Verlag; 2009. [Google Scholar]
- Decety J., Sommerville J.A. Shared representations between self and others: A social cognitive neuroscience view. Trends in Cognitive Sciences. 2003;7:527–33. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- DiPisapia N., Slomski J.A., Braver T.S. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cerebral Cortex. 2007;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- Dobbins I.G., Han S. Cue- versus probe- dependent prefrontal cortex activity during contextual remembering. Journal of Cognitive Neuroscience. 2006a;18:1439–52. doi: 10.1162/jocn.2006.18.9.1439. [DOI] [PubMed] [Google Scholar]
- Dobbins I.G., Han S. Isolating rule- versus evidence- based prefrontal activity during episodic and lexical discrimination: An fMRI investigation of detection theory distinctions. Cerebral Cortex. 2006b;16:1614–22. doi: 10.1093/cercor/bhj098. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. Development and neurophysiology of mentalising. Philosophical Transactions of the Royal Society of London B. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H., Hiraki K. Whose loss is it? Human electrophysiological correlates of non-self reward processing. Social Neuroscience. 2009;4:261–75. doi: 10.1080/17470910802625009. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J., Frith C.D., Burgess P.W. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. European Journal of Neuroscience. 2005;21:1423–31. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J., Spengler S., Simons J.S., Frith C.D., Burgess P.W. Differential functions of lateral and medial rostral prefrontal cortex (area 10) revealed by brain-behaviour correlations. Cerebral Cortex. 2006a;16:1783–9. doi: 10.1093/cercor/bhj113. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J., Spengler S., Simons J. S., et al. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience. 2006b;18:932–48. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J., Williamson I.D.M., Dumontheil I., Simons J.S., Frith C.D., Burgess P.W. Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Social Cognitive and Affective Neuroscience. 2007;2:217–26. doi: 10.1093/scan/nsm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A.E., Fugelsang J.A., Kraemer D.J., Shamosh N.A., Dunbar K.N. Frontopolar cortex mediates abstract integration in analogy. Brain Research. 2006;1096:125–37. doi: 10.1016/j.brainres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A. Being a self: considerations from functional imaging. Consciousness and Cognition. 2005;14:679–97. doi: 10.1016/j.concog.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R.N.A. Forward inference using functional neuroimaging: Dissociations versus associations. Trends in Cognitive Sciences. 2006;10:64–9. doi: 10.1016/j.tics.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D’Esposito M. Mentalizing about emotion and its relationship to empathy. Social Cognitive and Affective Neuroscience. 2008;3:204–17. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A.C., Macrae C.N., Mitchell J.P. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proceedings of the National Academy of Sciences USA. 2008;105:4507–12. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W.M., Macrae C.N., Wyland C.L., Caglar S., Inati S., Heatherton T.F. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Klucharev V., Hytönen K., Rijpkema M., Smidts A., Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–51. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Koechlin E., Corrado G., Pietrini P., Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proceedings of the National Academy of Sciences USA. 2000;97:7651–6. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E., Ody C., Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;14:1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kroger J.K., Sabb F.W., Fales C.L., Bookheimer S.Y., Cohen M.S., Holyoak K.J. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cerebral Cortex. 2002;12:477–85. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Krueger F., Barbey A.K., Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends in Cognitive Sciences. 2009;13:103–9. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Lamm C., Batson C.D., Decety J. The neural basis of human empathy - Effects of perspective-taking and cognitive appraisal: An event-related fMRI study. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Macrae C.N., Moran J.M., Heatherton T.F., Banfield J.F., Kelley W.M. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Vargha-Khadem F., Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124:1156–70. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Mechelli A., Henson R.N.A., Price C.J., Friston K.J. Comparing event-related and epoch analysis in blocked design fMRI. NeuroImage. 2003;18:806–10. doi: 10.1016/s1053-8119(02)00027-7. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Heatherton T.F., Macrae C.N. Distinct neural systems subserve person and object knowledge. Proceedings of the National Academy of Sciences USA. 2002;99:15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P., Banaji M.R., Macrae C.N. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Ames D.L., Jenkins A.C., Banaji M.R. Neural correlates of stereotype application. Journal of Cognitive Neuroscience. 2009;21:594–604. doi: 10.1162/jocn.2009.21033. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Knierim K., Ludlow D., Hanelin J., Ramachandran T., Mackey S. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1748–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Paxton J.L., Barch D.M., Racine C.A., Braver T.S. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18:1010–28. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27:11573–86. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack P.A., Mumford J.A. Independence in ROI analysis: where is the voodoo? Social Cognitive and Affective Neuroscience. 2009;4:208–13. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson L.T., Satpute A.B., Lieberman M.D. The neural correlates of implicit and explicit self-relevant processing. NeuroImage. 2010;50:701–8. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Ramnani N., Owen A.M. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–94. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Reynolds J.R., McDermott K.B, Braver T.S. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cerebral Cortex. 2006;16:519–28. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- Ruby P., Decety J. Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nature Neuroscience. 2001;4:546–50. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Ruby P., Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Rushton J.P., Chrisjohn R.D., Fekken G.C. The altruistic personality and the self-report altruism scale. Personality & Individual Differences. 1981;2:293–302. [Google Scholar]
- Seitz R.J., Nickel J., Azari N.P. Functional modularity of the medial prefrontal cortex: involvement in human empathy. Neuropsychology. 2006;20:743–51. doi: 10.1037/0894-4105.20.6.743. [DOI] [PubMed] [Google Scholar]
- Seger C.A., Stone M., Keenan J.P. Cortical activations during judgments about the self and an other person. Neuropsychologia. 2004;42:1168–77. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Shallice T., Burgess P.W. The domain of supervisory processes and the temporal organisation of behaviour. Philosophical Transactions of the Royal Society of London B. 1996;351:1405–12. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Simons J.S., Schölvinck M., Gilbert S.J., Frith C.D., Burgess P.W. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44:1388–97. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith R., Keramatian K., Christoff K. Functionally localizing the rostrolateral prefrontal cortex. NeuroImage. 2007;36:1387–96. doi: 10.1016/j.neuroimage.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Tankersley D., Stowe C.J., Huettel S.A. Altruism is associated with an increased neural response to agency. Nature Neuroscience. 2007;10:150–1. doi: 10.1038/nn1833. [DOI] [PubMed] [Google Scholar]
- Turner M.S., Simons J.S., Gilbert S.J., Frith C.D., Burgess P.W. Distinct roles for lateral and medial rostral prefrontal cortex in source monitoring of perceived and imagined events. Neuropsychologia. 2008;46:1442–53. doi: 10.1016/j.neuropsychologia.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Human Brain Mapping. 2009;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Jacoby L.L., Wheeler M.E., McAvoy M.P., Petersen S.E., Buckner R.L. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. Journal of Neuroscience. 2003;23:8460–70. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K., May M., Ritzl A., Falkai P.K., Zilles K., Fink G.R. Neural correlates of first-person perspective as one constituent of human self-consciousness. Journal of Cognitive Neuroscience. 2004;16:817–27. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Waltz J.A., Knowlton B.J., Holyoak K.J., et al. A system for relational reasoning in human prefrontal cortex. Psychological Science. 1999;10:119–25. [Google Scholar]
- Wendelken C., Donohue S.E., Crone E.A., Carter C.S., Bunge S.A. ‘Brain is to Thought as Stomach is to … ?’ – Specifying the role of rostrolateral prefrontal cortex in analogical reasoning. Journal of Cognitive Neuroscience. 2008;20:682–93. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Worsley K., Evans A., Marrett S., Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow & Metabolism. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zysset S., Huber O., Samson A., Ferstl E.C., von Cramon D.Y. Functional specialization within the anterior medial prefrontal cortex: a functional magnetic resonance imaging study with human subjects. Neuroscience Letters. 2003;335:183–6. doi: 10.1016/s0304-3940(02)01196-5. [DOI] [PubMed] [Google Scholar]