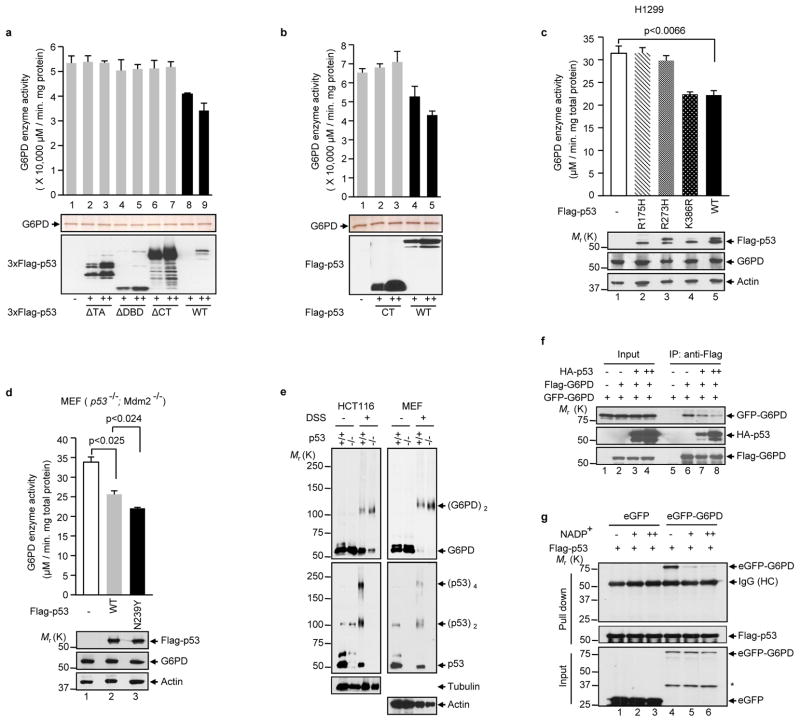
Figure 4. p53 inhibits the formation of dimeric G6PD holoenzyme.
(a, b) Activity of the G6PD protein after being incubated with purified wild type or mutant p53 proteins (top). Proteins were analyzed by silver staining (middle) and anti-Flag western blot (bottom). The p53 proteins were tagged with either three copies (a) or one copy (b) of the Flag epitope. Data are means ± S.D. (n=3).
(c, d) H1299 cells (c) and p53−/−Mdm2−/− MEFs (d) were transfected separately with wild type and mutant p53 proteins as indicated. G6PD activity (top) and protein expression (bottom) were analyzed. The K386R mutation blocks p53 SUMOylation. Data are means ± S.D. (n=3).
(e) Extracts of p53+/+ and p53−/− HCT116 and MEF were treated with and without 5 mM disuccinimidyl suberate (DSS) and analyzed by western blot with antibodies against G6PD, p53, and as controls, tubulin and actin. The positions of various forms of G6PD and p53 are indicated.
(f) H1299 cells were transfected with Flag-G6PD, eGFP-G6PD and different amounts of HA-p53. Cell lysates were incubated with anti-Flag antibody. Input and IP were analyzed by western blot.
(g) Lysates from p53−/− Mdm2−/− MEFs expressing GFP or GFP-G6PD were incubated with Flag-p53 immobilized on M2 beads or control beads in presence of increasing amounts of NADP+ (0, 0.1, and 1 mM). Input and beads-bound (pull down) proteins were analyzed by western blot.