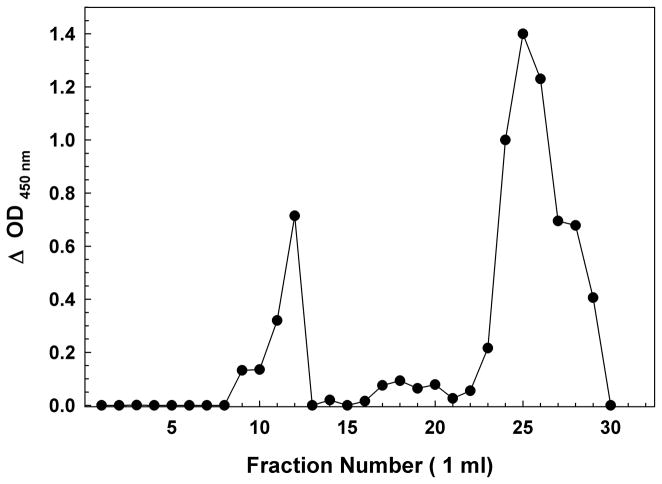
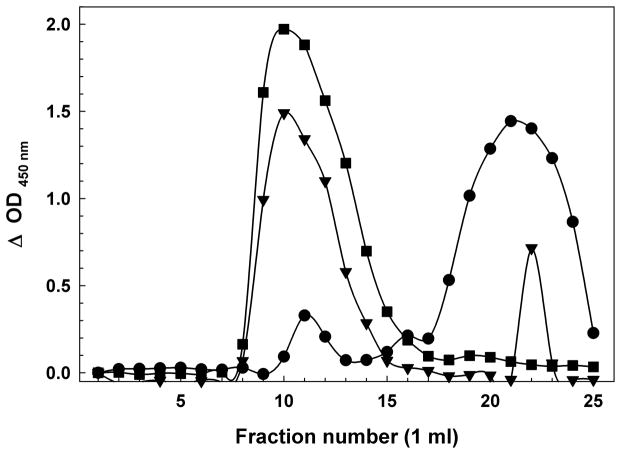
Figure 1.
(A) Sephadex G75 Size Exclusion Chromatography of a Synthetic Aβ(1-42) Oligomer preparation. The first peak of OD 450 nm (~fractions 9–12) represents the Aβ immunoreactivity of high molecular weight oligomeric Aβ eluting in the void volume, while the second peak (~fraction 23–30) represents primarily monomer and low-m.w. oligomers near the inclusion volume. BSA (66 kDa fr. 13); myoglobin (17 kDa fr. 20). (B) Sephadex G75 Size Exclusion Chromatography of H4 Neuroglioma-derived Aβ Peptides. Similar to the synthetic peptide, the first peak (~fractions 8–15) represents the Aβ immunoreactivity high molecular weight oligomeric Aβ eluting in the void volume, while the second peak (~fraction 18–25) represents primarily monomer and low-m.w. oligomers near the inclusion volume. BSA (66 kDa fr. 13); myoglobin (17 kDa fr. 20). As the H4 cells incubate in serum-free media, the population of Aβ in the media shifts from nearly all monomeric to largely high-m.w. oligomers. Day 1 (circles); Day 2 (inverted triangles); Day 3 (squares).