Table 2.
Scope of the photocatalytic [4+2] cycloaddition.a
| Entry | Methodb | Substrate | Product | Time | Yieldc |
|---|---|---|---|---|---|
 |
|||||
| 1 | A | Ar = Ph | 1 h | 86% | |
| 2 | A | Ar = 4-Cl-C6H4 | 30 min | 70% | |
| 3 | A | Ar = 4-CF3-C6H4 | 30 min | 83%d | |
| 4 | A | Ar = 4-AcO-C6H4 | 1 h | 76% | |
| 5 | A | Ar = 2-naphthyl | 1.5 h | 77% | |
| 6 | A | Ar = 2-furyl | 30 min | 77% | |
| 7 | A | Ar = 2-F-C6H4 | 30 min | 84% | |
| 8 | A | Ar = 2-Me-C6H4 | 6 h | 5% e | |
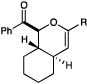 |
|||||
| 9 | A | R = CH2OBn | 20 min | 76% | |
| 10 | A | R = Me | 2 h | 39% | |
| 11 | B | R = Me | 10 min | 73% | |
| 12 | B | R = i-Pr | 1.5 h | 58% | |
| 13 | B | R = t-Bu | 6 h | 12%e | |
| 14 | B | 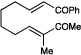 |
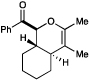 |
5 h | 17% e |
All reactions were irradiated with a 200 W tungsten filament light bulb at a distance of 30 cm.
Method A: Bis(enone) substrate (1 equiv), Ru(bpy)3Cl2(0.05 equiv), LiBF4 (2 equiv), i-Pr2NEt (3 equiv), and H2O (10 equiv) in degassed MeCN (0.1 M). Method B: Bis(enone) substrate (1 equiv), Ru(bpy)3Cl2 (0.05 equiv), Mg(ClO4)2 (2 equiv), and i-Pr2NEt (5 equiv) in degassed MeCN (0.025 M).
Data represent the average isolated yields from two reproducible experiments, unless otherwise noted.
Isolated yield of a single experiment.
Yield of a single experiment, determined by 1H NMR spectroscopy against an internal standard.
