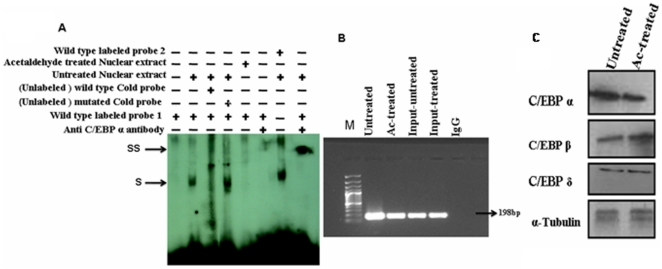
Figure 6. Acetaldehyde decrease/eliminates C/EBP alpha binding to CTSL promoter sequence.
(A) Demonstration of specific binding of C/EBP α to CTSL promoter. Radio-labeled double stranded DNA fragment (14 bp) encompassing wild type or mutated C/EBP α binding motif 1 or 2 ( described in figure - ) were incubated with the nuclear lysate (5 µg protein) prepared from acetaldehyde treated or untreated cells in the binding assay buffer. The DNA protein complex were resolved on non denaturing 5% PAGE and detected by autoradiography. The binding reactions were also carried out in the presence of 100 molar excess unlabelled double stranded DNA containing wild type or mutant C/EBP α binding motif to ascertain the specificity of DNA protein interaction. In some of the reactions 10 µg of antibody against C/EBP α were incubated with or without nuclear lysate before adding radio-labeled probe. Shift and supershift in the protein DNA complexes have been marked by S and SS respectively. (B) In vivo binding of C/EBP-α to CTSL promoter. Crosslinked chromatin isolated from acetaldehyde treated or untreated HepG2 cells using C/EBP-α antibody were subjected to PCR with gene specific primers (nucleotide sequence given in table) flanking the C/EBP-α binding motifs on CTSL promoter. The amplified products were resolved on agarose gel. The cross linked chromatin immunoprecipitated with normal rabbit IgG were also subjected to PCR using the same primers and served as negative control. Amplification of an expected 198 bp fragment is evident in all the lanes except negative control. Input control is from non immunoprecipitated total genomic DNA. (C) Effect of acetaldehyde treatment on levels of C/EBP transcription factors. Cell lysates of acetaldehyde treated or untreated cells were resolved on SDS-PAGE and subjected to western bloting using specific antibodies for C/EBPα, C/EBPβ or C/EBPδ as described in Materials and Methods section. Simultaneously western blot for α-Tubulin was performed and used for normalization for equal loading.