Abstract
Asperfuranone, a novel compound of genomic mining in Aspergillus nidulans, was investigated for its anti-proliferative activity in human non-small cell lung cancer A549 cells. To identity the anti-cancer mechanism of asperfuranone, we assayed its effect on apoptosis, cell cycle distribution, and levels of p53, p21 Waf1/Cip1, Fas/APO-1 receptor and Fas ligand. Enzyme-linked immunosorbent assay showed that the G0/G1 phase arrest might be due to p53-dependent induction of p21 Waf1/Cip1. An enhancement in Fas/APO-1 and its two form ligands, membrane-bound Fas ligand (mFasL) and soluble Fas ligand (sFasL), might be responsible for the apoptotic effect induced by asperfuranone. Our study reports here for the first time that the induction of p53 and the activity of Fas/Fas ligand apoptotic system may participate in the anti-proliferative activity of asperfuranone in A549 cells.
Lung cancer is the leading cause of cancer death in the world, and non-small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancer cases [1,2]. NSCLC commonly develop resistance to radiation and chemotherapy, and often present at stages too late for surgical intervention, resulting in a low overall survival at 5 years of less than 15% [3]. Since current treatment modalities are inadequate, novel therapies are needed to reduce the effects of the increasing incidence in pulmonary neoplasm.
Apoptosis plays an important role in homeostasis and development of the tissue in organism [4]. Imbalance between cell proliferation and apoptotic cell death will result in serious disease such as cancer. Many studies have demonstrated that cancer treatment by chemotherapy and γ-irradiation kill target cells primarily by the induction of apoptosis [4,5]. Several previous publications have reported that anti-cancer agents may induce apoptosis via the Fas/FasL system [6–11]. Fas is a cell surface receptor comprising a type1 integral membrane protein that expresses a cytoplasmic death domain and belongs to the tumour necrosis factor receptor superfamily [12,13]. Activation of Fas by its ligand (FasL) results in the oligomerization of its intracellular death domain and the recruitment of the intracellular adaptator Fas-associated death domain (FADD). Once bound, FADD is able to activate procaspases-8 and -10 in a death inducing signalling complex. In turn, caspases-8 and -10 activate downstream caspases, resulting in apoptotic cell death [14,15].
Asperfuranone (fig. 1), a novel compound of genomic mining in Aspergillus nidulans, was investigated for its anti-proliferative activity in human non-small cell lung cancer A549 cells. Furthermore, to establish the anti-cancer mechanism of asperfuranone, we assayed the levels of p53, p21 Waf1/Cip1, Fas/APO-1 receptor, and Fas ligand, which are strongly associated with the signal transduction of apoptosis and affect the chemo-sensitivity of tumour cells to anti-cancer agents.
Fig. 1.
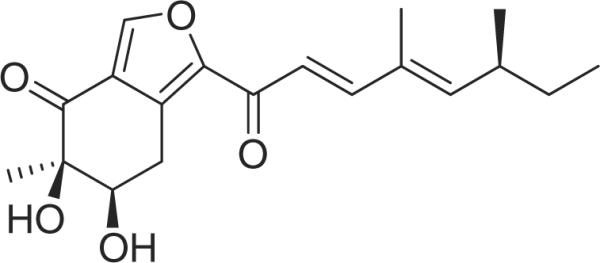
Chemical structure of asperfuranone from Aspergillus nidulans.
Materials and Methods
Isolation and identification of secondary metabolites
Asperfuranone was purified as described before [16]. Briefly, alcA(p)-afoA A. nidulans was grown in 3 l of LMM medium (1 × 106 spores/ml). After 18 hr, cyclopentanone at 30 mM was added to induce afoA gene, a positive regulator for asperfuranone biosynthesis. After 48 hr of induction, culture medium was collected by filtration and partitioned with EtOAc twice. The mycelium which also containing asperfuraone was extracted separately with 500 ml MeOH twice, with each sonicated for 1 hr. Both extracts were pooled to get 164.3 mg of crude extract. This extract was then applied to a SiO2 gel column (Merck 230–400 mesh, ASTM, 20 × 60 mm) and eluted with CHCl3–MeOH mixtures of increasing polarity (fraction A, 1:0, 200 ml; fraction B, 19:1, 200 ml; fraction C, 9:1, 200 ml; fraction D, 7:3, 200 ml). Fraction C (67.3 mg), which contained asperfuraonone, was further purified by reverse phase HPLC [Phenomenex Luna 5 lm C18 (2), 250 × 10 mm] with a flow rate of 5.0 ml/min. and measured by a UV detector at 254 nm. The gradient system was MeCN (solvent B) in 5% MeCN/H2O (solvent A) both containing 0.05% TFA: 42–45% B from 0–20 min., 45–100% B from 20–26 min., maintained at 100% B from 26–31 min., 100–42% B from 31–32 min., and re-equilibration with 42% B from 32–37 min. Asperfuranone (32.8 mg) was eluted at 15.0 min. The stock solution of asperfuranone was prepared at a concentration of 80 mM of dimethyl sulfoxide (DMSO). It was then stored at −20°C until use. For all experiments, the final concentrations of the test compound were prepared by diluting the stock with DMEM. Control cultures received the carrier solvent (0.1% DMSO).
Reagents and materials
Foetal calf serum (FCS), penicillin G, streptomycin, amphotericin B and RPMI 1640 were obtained from GIBCO BRL (Gaithersburg, MD, USA). Dimethyl sulfoxide, ribonuclease (RNase), and propidium iodide (PI) were purchased from Sigma Chemical (St Louis, MO, USA). XTT ELISA kit was obtained from Roche Diagnostics GmbH (Mannheim, Germany). p53 ELISA, Fas Ligand ELISA, and caspase-8 assay kits, and caspase-8 inhibitor, benzyloxy-carbonyl-Val-Ala-Asp-fluoromethylke-tone (Z-IETD-FMK) were purchased from Calbiochem (Cambridge, MA, USA). p21 Waf1/Cip 1 and Fas/APO-1 ELISA kits were purchased from Invitrogen (Camarillo, CA, USA). Anti-Fas Ab (ZB4) was obtained from Upstate Biotechnology, Inc. (Lake Placid, NY, USA).
Cell culture
Human non-small cell lung carcinoma cell line, A549 (American Type Culture Collection [ATCC] CCL185), human hepatocellular carcinoma cell line, PLC/PRF/5 (ATCC CRL 8024), and human breast adenocarcinoma cell line, MDA-MD-231 cells (ATCC HTB-26) were maintained in monolayer cultures at 37°C and 5% CO2 in RPMI 1640 supplemented with 10% FCS, 0.1 mg/ml streptomycin, and 100 units/ml penicillin (Life Technologies, Inc., Grand Island, NY, USA).
Cell proliferation
Inhibition of cell proliferation by asperfuranone was measured by XTT (sodium 3'-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate) assay. Briefly, cells were plated in 96-well culture plates (1 × 104 cells/well, and after 24 hr incubation, treated with vehicle alone (0.1% DMSO) and various concentrations of asperfuranone for 48 hr. All cell proliferation data were analysed in triplicate for different concentrations. 150 μl XTT test solution, which was prepared by mixing 5 ml of XTT-labelling reagent with 100 μl of electron coupling reagent, was then added to each well. After 4 hr of incubation, absorbance was measured on an ELISA reader (Multiskan EX; Labsystem Multiskan, Helsinki, Finland) at a test wavelength of 492 nm and a reference wavelength of 690 nm. The data were expressed as a percentage of the absorbance of control (% survival). The IC50 value was defined as the concentration of asperfuranone causing a 50% reduction in absorbance compared with control. IC50 was obtained using the non-linear regression programme GraphPad Prism software fitted to the plot of % survival versus concentrations.
Cell cycle analysis
To determine cell cycle distribution, 5 × 105 cells were plated in 60-mm dishes and treated vehicle alone (0.1% DMSO) and asperfuranone (15 and 20 μM) for 24 hr. After treatment, the cells were collected by trypsinization, fixed in 70% ethanol, washed in PBS, re-suspended in 1 ml of PBS containing 1 mg/ml RNase and 50 μg/ml propidium iodide, incubated in the dark for 30 min. at room temperature and analysed by EPICS flow cytometer. The data were analysed using the Multicycle software (Phoenix Flow Systems, San Diego, CA, USA).
Apoptosis assay
We provided a quantitative evaluation by using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick endlabelling (TUNEL) method to examine DNA-strand breaks during apoptosis. Briefly, cells were incubated with vehicle alone (0.1% DMSO) and asperfuranone (15 and 20 μM) for the indicated times. The cells were trypsinized, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate. After being washed, the cells were incubated with the reaction mixture provide by BD ApoAlert™ DNA Fragmentation Assay kit for 60 min. at 37°C. The stained cells were then analysed with an EPICS flow cytometer and a fluorescence microscope at 20 × magnification.
Assaying the levels of p53, p21 Waf1/Cip1, Fas/APO-1 and Fas ligand (mFasL and sFasL)
p53 ELISA, p21 Waf1/Cip1, Fas/APO-1 ELISA and Fas Ligand ELISA kits were used to detect p53, p21 Waf1/Cip1, Fas/APO-1 receptor and soluble (sFasL)/membrane-bound Fas ligand (mFasL). Briefly, A549 cells were treated with vehicle alone (0.1% DMSO) and asperfuranone (15 and 20 μM) for 6, 12, 24 and 48 hr. The samples of cell lysate were placed in 96-well microtitre plates that were coated with monoclonal detective antibodies, and incubated at room temperature. Each sample was assessed in triplicate. It was necessary to determine the soluble Fas ligand in cell culture supernatant by using Fas Ligand ELISA kit. After removing the unbound material by washing with washing buffer (50 mM Tris, 200 mM NaCl, and 0.2% Tween 20), the detector antibody that is bound by horseradish peroxidase, conjugated streptavidin, was added to bind to the antibodies. Horseradish peroxidase catalyzed the conversion of a chromogenic substrate (tetramethylbenzidine) to a coloured solution with colour intensity proportional to the amount of protein present in the sample. The absorbance of each well was measured at 450 nm, and concentrations of p53, p21 Waf1/Cip1, Fas/APO-1 and FasL were determined by interpolating from standard curves obtained with known concentrations of standard proteins.
Assay for caspase-8 activity
The assay is based on the ability of the active enzyme to cleave the chromophore from the enzyme substrate, Ac-IETD-pNA. The cell lysates were incubated with peptide substrate in assay buffer (100 mM NaCl, 50 mM HEPES, 10 mM dithiothreitol, 1 mM EDTA, 10% glycerol, 0.1% CHAPS, pH 7.4) for 3 hr at 37°C. Each sample was assayed in triplicate. The release of p-nitroaniline was monitored at 405 nm. Results are represented as the percent change of the activity compared to the untreated control.
Statistical analysis
Data were expressed as mean ± S.D. Statistical comparisons of the results were made using ANOVA. Significant differences (p < 0.05) between the means of control and asperfuranone-treated cells were analysed by Dunnett'stest.
Results
Asperfuranone inhibits cell proliferation in A549 cells
We first tested the anti-proliferative effect of asperfuranone in human non-small-cell lung cancer A549 cells. As shown in fig. 2, the growth inhibitory effect of asperfuranone was observed in a dose-dependent manner. At 48 hr, the maximal effect on proliferation inhibition was observed with 20 μM asperfuranone, which inhibited proliferation in 64.5% of A549 cells. The IC50 values were 15.3 μM.
Fig. 2.
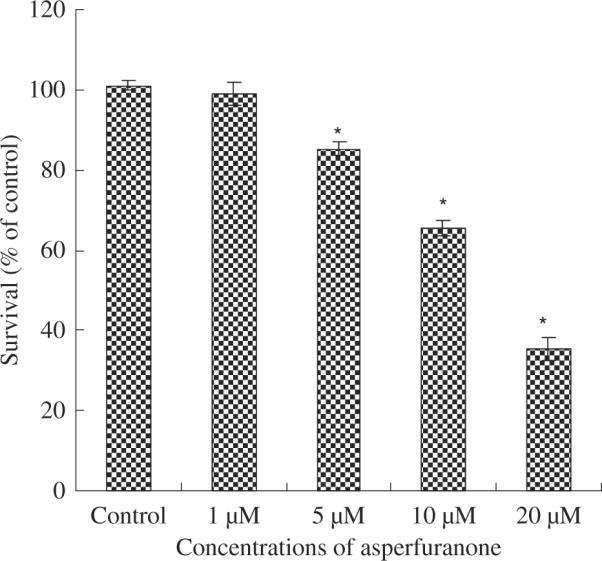
The inhibitory effects of asperfuranone on cell proliferation inhibition in non-small-cell lung cancer A549 cells. Cell growth inhibition activity of asperfuranone was assessed by XTT. Results are expressed as the percentage of cell proliferation relative to the proliferation of control. The data shown are the mean from three independent experiments. Each value is the mean ± s.d. of three determinations. The asterisk indicates a significant difference between control and asperfuranone-treated cells, as analysed by Dunnett's test(p < 0.05).
We also assessed the effect of asperfuranone on two other human cancer cell lines: PLC/PRF/5 (liver cancer) and MDA-MB-231 (breast cancer). The results revealed that asperfuranone also inhibited the proliferation of these cancer cell lines. The IC50 values were 18.6 and 16.5 μM for PLC/PRF/5 and MDA-MB-231, respectively (data not shown).
Asperfuranone-induced cell cycle arrest and apoptosis in A549 cells
The results on the effect of asperfuranone on cell cycle progression of A549 are shown in fig. 3. As compared to the control, 15 μM of asperfuranone increased the population of G0/G1 phase from 30.7% to 52.6%. This effect was enhanced when A549 cells were treated by 20 μM of asperfuranone (72.3% cell population in G0/G1 phase).
Fig. 3.
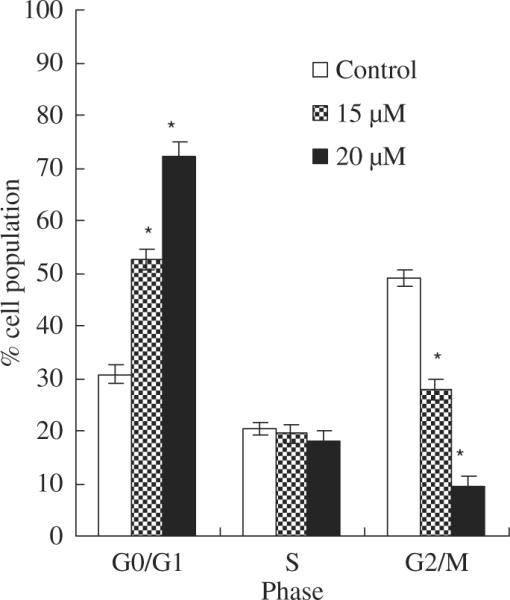
The effects of asperfuranone on cell cycle distribution in A549 cells. A549 cells after treatment with vehicle alone (0.1% DMSO) and asperfuranone (15 and 20 μM) for 24 hr were fixed and stained with propidium iodide, and cell cycle distribution was then analysed by flow cytometry. Each value is the mean ± S.D. of three determinations. The asterisk indicates a significant difference between control and asperfuranone-treated cells, as analysed by Dunnett's test(p < 0.05).
We next assessed the effect of asperfuranone on the induction of apoptosis in A549 cells. A quantitative evaluation was made using TUNEL to detect DNA-strand breaks. Compared to vehicle-treated cells, asperfuranone induced 36.33% of apoptotic cells in A549 cells at concentrations of 20 μM at 48 hr (fig. 4).
Fig. 4.
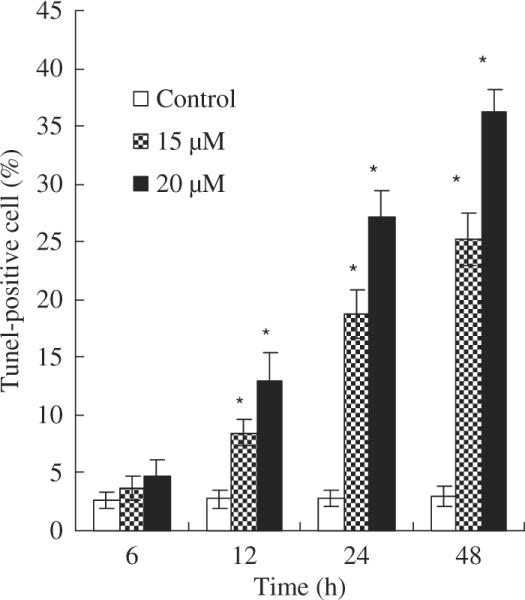
Induction of apoptosis in A549 cells by asperfuranone. The effect of asperfuranone in A549 cells. A549 cells were treated with vehicle alone (0.1% DMSO) and various concentrations of asperfuranone for the indicated times, and then apoptotic cells were stained by TUNEL assay. The TUNEL-positive cells were examined by flow cytometry. Each value is the mean ± s.d. of three determinations. The asterisk indicates a significant difference between control and asperfuranone-treated cells, as analysed by Dunnett's test (p < 0.05).
Asperfuranone increases the expression of p53 and p21 Waf1/Cip1 proteins in A549 cells
To determine whether tumour suppression factor p53 are involved in the asperfuranone-mediated anti-proliferative effect of A549 cells, the levels of p53 protein were assayed by ELISA. In the study of p53 protein expression was treated with 15 and 20 μM asperfuranone for 6, 12, 24 and 48 hr. Marked induction of p53 protein was observed in a dose-dependent manner (fig. 5A). The up-regulation of p53 by asperfuranone started to increase 6 hr after treatment with asperfuranone and reached maximum expression at 12 hr.
Fig. 5.
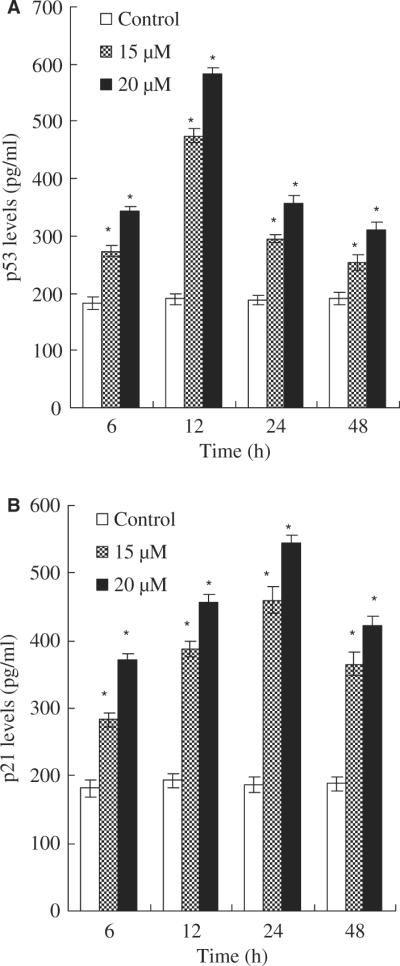
Effects of asperfuranone on protein expression of p53 and p21 Waf1/Cip1. The level of p53 protein (A); the level of p21 Waf1/Cip1 (B). A549 cells were treated with vehicle alone (0.1% DMSO) and asperfuranone (15 and 20 μM). p53 and p21 Waf1/Cip1 levels were determined by p53 and p21 Waf1/Cip1 ELISA kits, respectively. The detailed protocol is described in `Materials and Methods'. Each value is the mean ± S.D. of three determinations. The asterisk indicates a significant difference between control and asperfuranone-treated cells, as analysed by Dunnett's test(p < 0.05).
The p21 Waf1/Cip1 protein is first characterized as a downstream target of p53 and is thought to be responsible for G0/G1 cell cycle arrest [17]. Thus, we also assessed the p21 Waf1/Cip1 expression of p53-expressing A549 cells performed with a p21 Waf1/Cip1 immunoassay. Figure 5B shows that an increase in p21 Waf1/Cip1 protein was apparent at 6 hr and reached maximum induction at 24 hr in asperfuranone-treated A549 cells. Moreover, the induction of p21 Waf1/Cip1 was in a dose-dependent manner. Based on these data, we suggest that asperfuranone-mediated cell cycle arrest might operate through the induction of p21 Waf1/-Cip1 protein on a p53-dependent event in A549 cells.
Fas/FasL apoptotic system might be a possible pathway of asperfuranone-mediated apoptosis
By using Fas/APO-1 and Fas Ligand ELISA kits, we found that asperfuranone increased expression of Fas/APO-1 receptor and soluble/membrane-bound Fas ligand in A549 cells as early as 6 hr after treatment in a dose-dependent fashion (fig. 6). The maximum effect was observed after 24 hr of treatment. The time relationship between the expression of Fas/FasL at 6 hr of treatment and the occurrence of apoptosis at 12 hr of treatment could support the idea that the Fas/FasL system might mediate asperfuranone-induced apoptosis of A549 cells.
Fig. 6.
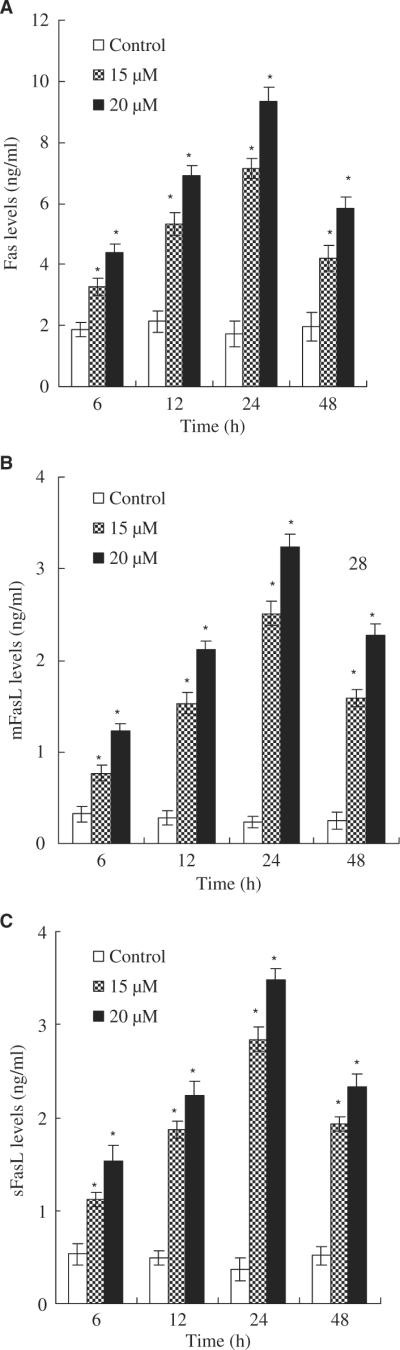
Fas/FasL apoptotic system was involved in asperfuranone-mediated apoptosis. A549 cells were incubated with vehicle alone (0.1% DMSO) and asperfuranone (15 and 20 μM) for 6, 12, 24 and 48 hr. The level of Fas/APO-1 receptor (A); the amount of mFasL (B); the amount of sFasL (C). Each value is the mean ± s.d. of three determinations. The asterisk indicates a significant difference between control and asperfuranone-treated cells, as analysed by Dunnett's test(p < 0.05).
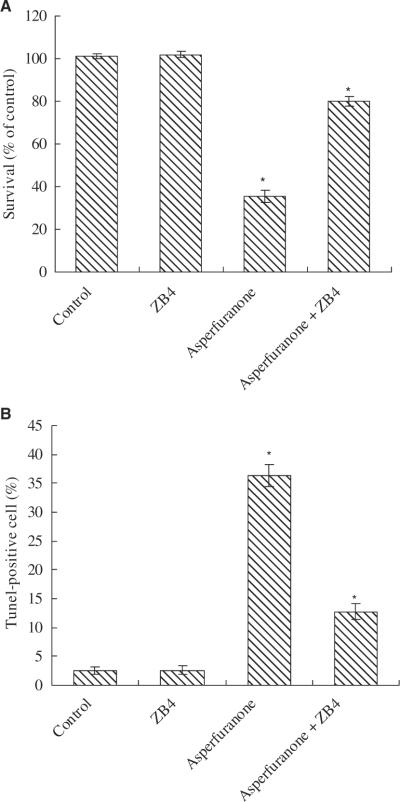
When A549 cells were pre-treated with an antagonistic anti-Fas antibody, ZB4, the anti-proliferative and pro-apoptotic effects of asperfuranone were effectively inhibited. At 20 μM of asperfuranone, cell proliferation inhibition decreased from 64.5% to 19.8% in A549, respectively (fig. 7A). Compared to the control, the induction of apoptosis induced by 20 μM of asperfuranone decreased from about 36.33% to 12.74% at 48 hr in ZB4 pre-treated A549 cells (fig. 7B).
Fig. 7.
Effect of antagonistic anti-Fas antibody (ZB4) on asperfuranone in A549 cells. (A) The anti-proliferative and (B) the pro-apoptotic effect of asperfuranone was decreased by Fas antagonist ZB4. For blocking experiments, cells were pre-incubated with 250 ng/ml ZB4 for 1 hr and then treated with 20 μM of asperfuranone for 48 hr. Cell viability and apoptosis induction were examined by XTT and BD ApoAlert™ DNA Fragmentation Assay kit. The data shown are the mean ± S.D. of three determinations. The asterisk indicates a significant difference between control and asperfuranone-treated cells, as analysed by Dunnett's test(p < 0.05).
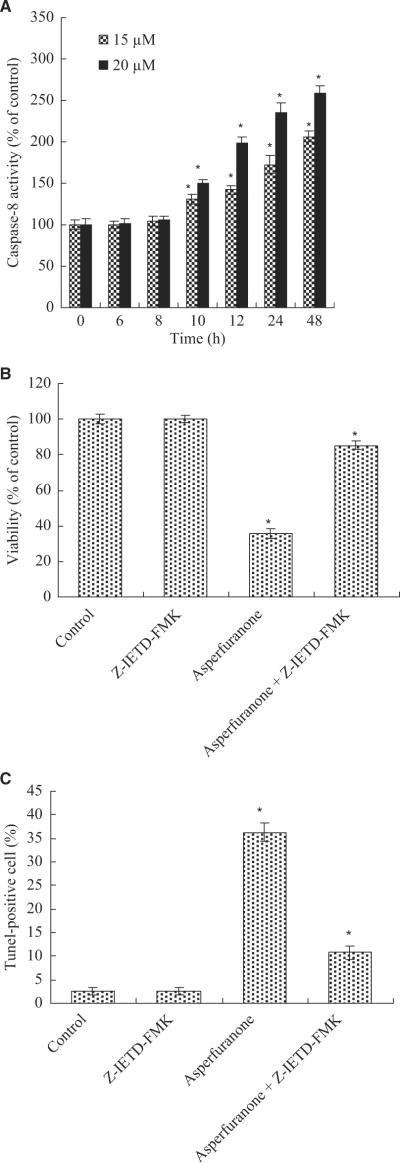
We next measured the downstream caspase of Fas/FasL system. The results showed that caspase-8 activity increased at 10 hr and reached maximum induction at 48 hr in 20 μM asperfuranone-treated A549 cells (fig. 8A). The activation of caspase-8 (at 10 hr) was before the production of DNA fragmentation (at 12 hr) showing caspase-8 activation was required in asperfuranone-induced apoptosis. To further provide this hypothesis, we assessed the role of caspase-8 on asperfuranone-mediated anti-proliferative and pro-apoptotic effects by using caspase-8 inhibitor (Z-IETD-FMK). Our results showed that inhibition of caspase-8 not only decreased the anti-proliferative activity of asperfuranone (fig. 8B), but also completely abolished induction of apoptosis in A549 cells (fig. 8C).
Fig. 8.
(A) The activation of caspase-8 in A549 cells by asperfuranone; (B) Effect of caspase-8 inhibitor on asperfuranone-mediated anti-proliferation; (C) Effect of caspase-8 inhibitor on asperfuranone-induced apoptosis. A549 cells were incubated with various concentrations of asperfuranone for the indicated times. For blocking experiments, cells were pre-incubated with Z-IETD-FMK (10 μM) for 1 hr before the addition of 20 μM asperfuranone. After 48 hr of treatment, cell viability and induction of apoptosis were measured by XTT and BD ApoAlert™ DNA Fragmentation Assay kit. Each value is the mean ± s.d. of three determinations. The asterisk indicates a significant difference between control and asperfuranone-treated cells, as analysed by Dunnett's test(p < 0.05).
Discussion
Normal p53 gene is well-known to play a crucial role in inducing apoptosis and acting as cell cycle checkpoints in human and murine cells after DNA damage [17]. p21 Waf1/-Cip1 protein blocks the activities of various cyclin-dependent kinases [18–20], and inhibits the phosphorylation of retinoblastoma (RB) protein, thereby preventing the G1–S phase transition [19,21]. Previous studies have shown that p21 Waf1/Cip1 is transcriptionally regulated by p53-dependent and -independent pathways [22–24]. Our results demonstrate that p53 plays an important role in asperfuranone-induced anti-proliferative activity in A549 cells. Induction of p53 by asperfuranone can not only arrest cell cycle, but also trigger apoptosis in A549 cells. This finding is supported by the following results: first, flow cytometry assay indicated that asperfuranone arrested the cell cycle in the G0/G1 phase, which was attributed to the enhancement of p21 Waf1/Cip1 protein that might be induced by p53. Second, pro-apoptotic downstream target of p53, Fas/APO-1 protein [13] was increased by asperfuranone. Moreover, the expression levels of these proteins were greatly increased (at 24 hr) after maximal accumulation of p53 protein (at 12 hr) in A549 cells.
Fas/FasL system is a key signalling transduction pathway of apoptosis in cells and tissues [13]. Kim et al. have demonstrated that delivery of the wild-type murine Fas-associating death domain protein gene to drug- and radiation-resistant lung cancer may be a novel method for therapy of non-small cell lung cancer [15]. Ligation of Fas by agonistic antibody or its mature ligand induces receptor oligomerization and formation of death-inducing signalling complex (DISC), followed by activation of caspase-8, then further activating a series caspase cascades resulting in cell apoptotic death [13,25]. FasL is a TNF-related type II membrane protein [26]. Cleavage of membrane-bound Fas ligand by a metallo-protease-like enzyme results in the formation of soluble Fas ligand [27]. Fas is expressed in human airway epithelial cells and plays a critical role in the pathophysiology of various pulmonary disorders [28]. Up-regulation of Fas expression has been demonstrated to induce apoptosis in hydrogen peroxide-treated A549 cells [28]. Induction of Fas expression and augmentation of Fas/Fas ligand-mediated apoptosis has been also suggested to inhibit proliferation in the synthetic retinoid CD437-treated human non-small cell lung cancer cells [10]. Serrao et al. have reported that neutrophils induce apoptosis of lung epithelial cells via release of soluble FasL [29]. Our study indicated that Fas ligands, mFasL and sFasL, increased in asperfuranone-treated A549 cells. Moreover, the levels of Fas and the activity of caspase-8 were simultaneously enhanced in FasL-up-regulating A549 cells. Furthermore, when the Fas/Fas ligand system was blocked by ZB4, a decrease in cell growth inhibition and the pro-apoptotic effect of asperfuranone was noted. Similarly, cell growth inhibition and apoptotic induction of asperfuranone decreased in A549 cells treated with caspase-8 inhibitor. Thus, these findings are the first to show that the Fas/FasL system plays an important role in asperfuranone-mediated A549 cellular apoptosis.
In summary, our study suggests that the induction of p53 and activity of the Fas/FasL apoptotic system may participate in the anti-proliferative activity of asperfuranone in A549 cells. Our data provide a basic mechanism for the pro-apoptotic properties of asperfuranone in lung cancer cells.
Acknowledgements
This work was funded in part by Grant Number PO1GM084077 from the National Institute of General Medical Sciences to CCCW.
References
- 1.Gajra A, Newman N, Gamble GP, Abraham NZ, Kohman LJ, Graziano SL. Impact of tumor size on survival in stage IA non-small cell lung cancer: a case for subdividing stage IA disease. Lung Cancer. 2003;42:51–7. doi: 10.1016/s0169-5002(03)00285-x. [DOI] [PubMed] [Google Scholar]
- 2.McCracken M, Olsen M, Chen MS, Jr, Jemal A, Thun M, Cokkinides V, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57:190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- 3.Erridge SC, Møller H, Price A, Brewster D. International comparisons of survival from lung cancer: pitfalls and warnings. Nat Clin Pract Oncol. 2007;4:570–7. doi: 10.1038/ncponc0932. [DOI] [PubMed] [Google Scholar]
- 4.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–88. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 5.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 6.Chang GC, Yu CT, Tsai CH, Tsai JR, Chen JC, Wu CC, et al. An epidermal growth factor inhibitor, Gefitinib, induces apoptosis through a p53-dependent upregulation of pro-apoptotic molecules and downregulation of anti-apoptotic molecules in human lung adenocarcinoma A549 cells. Eur J Pharmacol. 2008;600:37–44. doi: 10.1016/j.ejphar.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Du A, Zhao B, Miao J, Yin D, Zhang S. Safrole oxide induces apoptosis by up-regulating Fas and FasL instead of integrin beta4 in A549 human lung cancer cells. Bioorg Med Chem. 2006;14:2438–45. doi: 10.1016/j.bmc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Rho JK, Choi YJ, Ryoo BY, Na II, Yang SH, Kim CH, et al. p53 enhances gefitinib-induced growth inhibition and apoptosis by regulation of Fas in non-small cell lung cancer. Cancer Res. 2007;67:1163–9. doi: 10.1158/0008-5472.CAN-06-2037. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Kondo M, Ito Y, Kume H, Suzuki R, Yamaki K. Soluble Fas and Fas ligand provide new information on metastasis and response to chemotherapy in SCLC patients. Cancer Detect Prev. 2005;29:175–80. doi: 10.1016/j.cdp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Sun SY, Yue P, Hong WK, Lotan R. Induction of Fas expression and augmentation of Fas/Fas ligand-mediated apoptosis by the synthetic retinoid CD437 in human lung cancer cells. Cancer Res. 2000;60:6537–43. [PubMed] [Google Scholar]
- 11.Yoshimoto Y, Kawada M, Ikeda D, Ishizuka M. Involvement of doxorubicin-induced Fas expression in the antitumor effect of doxorubicin on Lewis lung carcinoma in vivo. Int Immunopharmacol. 2005;5:281–8. doi: 10.1016/j.intimp.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Chopin V, Toillon RA, Jouy N, Le Bourhis X. Sodium butyrate induces P53-independent, Fas-mediated apoptosis in MCF-7 human breast cancer cells. Br J Pharmacol. 2002;135:79–86. doi: 10.1038/sj.bjp.0704456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 14.Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol. 1998;8:1001–8. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- 15.Kim PK, Park SY, Koty PP, Hua Y, Luketich JD, Billiar TR. Fas-associating death domain protein overexpression induces apoptosis in lung cancer cells. J Thorac Cardiovasc Surg. 2003;125:1336–42. doi: 10.1016/s0022-5223(02)73227-3. [DOI] [PubMed] [Google Scholar]
- 16.Chiang YM, Szewczyk E, Davidson AD, Keller N, Oakley BR, Wang CC. A gene cluster containing two fungal polyketide synthases encodes the biosynthetic pathway for a polyketide, Asperfuranone, in Aspergillus nidulans. J Am Chem Soc. 2009;131:2965–70. doi: 10.1021/ja8088185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 1999;18:7621–36. doi: 10.1038/sj.onc.1203285. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–10. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- 19.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 20.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 21.Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, et al. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–23. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 22.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 23.Michieli P, Chedid M, Lin D, Pierce JH, Mercer E, Givol D. Induction of Waf1/CIP1 by a p53-independent pathway. Cancer Res. 1994;54:3391–5. [PubMed] [Google Scholar]
- 24.Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, et al. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–44. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 25.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 26.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–78. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 27.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita T, Maruyama M, Araya J, Sassa K, Kawagishi Y, Hayashi R, et al. Hydrogen peroxide induces upregulation of Fas in human airway epithelial cells via the activation of PARP-p53 pathway. Am J Respir Cell Mol Biol. 2002;27:542–52. doi: 10.1165/rcmb.4775. [DOI] [PubMed] [Google Scholar]
- 29.Serrao KL, Fortenberry JD, Owens ML, Harris FL, Brown LA. Neutrophils induce apoptosis of lung epithelial cells via release of soluble Fas ligand. Am J Physiol Lung Cell Mol Physiol. 2001;280:L298–305. doi: 10.1152/ajplung.2001.280.2.L298. [DOI] [PubMed] [Google Scholar]