Scheme 4.
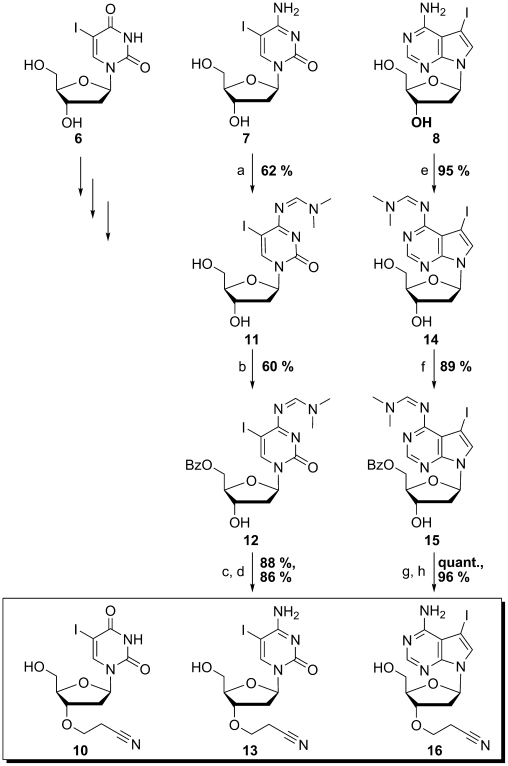
Protecting-group strategy and introduction of the 3′-modification into 6,[22] 7, and 8. Reagents and conditions: a) N,N-dimethylformamide dimethylacetal, dry DMF, 55 °C, 2.5 h; b) BzCl, dry pyridine/dry DMF=4:1, 0 °C→rt, 2 h; c) acrylonitrile, Cs2CO3, tBuOH/dry DMF 2:1, rt, 3 h; d) saturated methanolic ammonia, rt, 2.5 h; e) N,N-dimethylformamide dimethyl acetal, dry DMF, 50 °C, 2 h; f) BzCl, dry CH2Cl2, dry pyridine, −15 °C, 1 h; g) acrylonitrile, Cs2CO3, tBuOH, rt, 2 h; h) saturated methanolic ammonia, 50 °C, 2 h. Bz=benzoyl, BzCl=benzoyl chloride.
