Scheme 5.
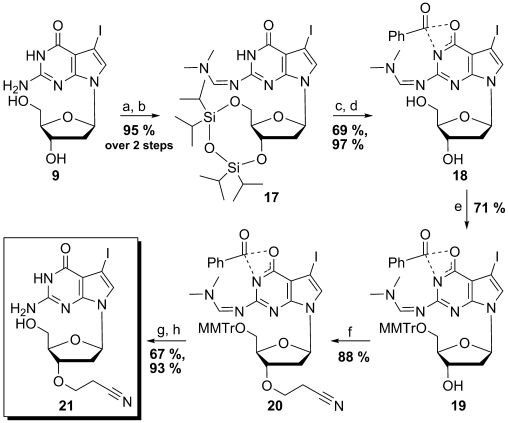
Protecting-group strategy and introduction of the 3′-modification into 9. Reagents and conditions: a) 1,1,3,3-tetraisopropyldichlorodisiloxane, dry pyridine, 0 °C→rt, 1 h; b) N,N-dimethylformamide dimethylacetal, dry DMF, rt, 24 h; c) BzCl, dry pyridine, dry CH2Cl2, 0 °C→rt, 2 h; d) Et3N⋅3 HF, THF, rt, 1 h; e) MMTrCl, DMAP, dry pyridine, rt, 18 h; f) acrylonitrile, Cs2CO3, tBuOH, rt, 2 h; g) PTSA, CH2Cl2/EtOH 1:1, rt, 1 h; h) 32 % aqueous NH3, MeOH, rt, 18 h. DMAP=N,N-dimethylaminopyridine, MMTr=monomethoxytrityl, PTSA=para-toluenesulfonic acid.
