Abstract
Complications of intrauterine growth restriction (IUGR) include increased pulmonary morbidities and impaired alveolar development. Normal alveolar development depends upon elastin expression and processing, as well as the formation and deposition of elastic fibers. This is true of the human and rat. In this study, we hypothesized that uteroplacental insufficiency (UPI)-induced IUGR decreases mRNA levels of elastin and genes required for elastin fiber synthesis and assembly, at birth (prealveolarization) and postnatal day 7 (midalveolarization) in the rat. We further hypothesized that this would be accompanied by reduced elastic fiber deposition and increased static compliance at postnatal day 21 (mature lung). We used a well characterized rat model of IUGR to test these hypotheses. IUGR decreases mRNA transcript levels of genes essential for elastic fiber formation, including elastin, at birth and day 7. In the day 21 lung, IUGR decreases elastic fiber deposition and increases static lung compliance. We conclude that IUGR decreases mRNA transcript levels of elastic fiber synthesis genes, before and during alveolarization leading to a reduced elastic fiber density and increased static lung compliance in the mature lung. We speculate that the mechanism by which IUGR predisposes to pulmonary disease may be via decreased lung elastic fiber deposition.
Keywords: intrauterine growth restriction, lung development, elastin
intrauterine growth restriction (IUGR) refers to the failure of a fetus to achieve its genetically predetermined size (14, 63). In developed countries, IUGR commonly results from maternal uteroplacental insufficiency (UPI) that occurs in association with vascular disorders such as pre-eclampsia (13, 25, 49). IUGR is a predisposition for preterm birth and occurs in 5–12% of premature births in the United States (18). Complications of IUGR include increased pulmonary morbidities. In human preterm infants, IUGR increases the risk and severity of the chronic lung disease of infancy (bronchopulmonary dysplasia, BPD) (4, 15, 46, 48). In term infants, IUGR increases the need for respiratory support (17, 34, 41–42, 58). Despite these complications, the characteristics of the IUGR lung that predispose to disease have not been fully elucidated.
The IUGR lung consistently displays impaired alveolar development in animal models (16, 23, 38–39, 43). We have previously shown increased lung mesenchymal thickness in UPI-induced IUGR rats at birth, a time when the rat lung is in the saccular stage of development (43). Others have demonstrated reduced alveolar number in rats rendered IUGR by maternal food restriction (23). In the sheep, where the lung is developmentally more mature at term, IUGR is also associated with impaired alveolar development (16, 38–39).
Normal alveolar development depends upon elastin expression and processing, as well as the formation and deposition of elastic fibers (11, 32, 37, 40, 62). In the mammalian lung, elastin fibers are distributed extensively within the alveolar walls, contributing to the structural integrity and the distensability of airspaces (11, 32, 37, 40, 62). The organization of lung elastin fibers begins early in the development of the lung and increases through the saccular stage, peaking during alveolarization (56). Elastin fiber deposition depends not only on the presence of elastin, but on the coordinated expression of a host of genes, including transforming growth factor (TGF)-α, TGF-β, fibrillin, fibulin, and lysyl oxidase (7, 36, 60).
Disruptions in elastin deposition effect alveolarization and lung function. Rat pups exposed to postnatal hyperoxia have decreased elastin expression, disruption of elastin fibers, increased static compliance and an arrest of alveolarization (10, 12, 47). The importance of a critical level of elastin deposition during lung development has been exemplified by studies examining mice expressing variable levels of elastin. Mice lacking elastin (Eln−/−) have arrested terminal airway branching with fewer distal air sacs (62). In contrast, lungs of heterozygous elastin-deleted (Eln+/−) mice (lung elastin ∼45% lower than wild type) are morphologically similar to control lungs (51). However, static compliance, a measure of the ability of the lungs to distend in response to pressure, is increased in Eln+/− mice. Another important consideration is that Eln+/− lungs are more susceptible to developing emphysema in response to cigarette smoke than control lungs (51). These finding imply that a critical level of lung elastin during development is required for appropriate lung development and that even a modest reduction in elastin confers functional differences and a greater susceptibility to lung damage (51).
Despite impaired alveolar development in IUGR and the importance of elastin in alveolar formation, the effect of IUGR on elastin deposition in the rat lung is unknown. An understanding of elastin deposition in the IUGR lung will help elucidate potential mechanisms by which IUGR causes a predisposition to lung disease. In this study, we hypothesized that UPI-induced IUGR decreases mRNA levels of elastin and genes required for elastin fiber synthesis and assembly, at birth and postnatal day 7. We further hypothesized that this would be accompanied by reduced elastin deposition and increased static compliance at postnatal day 21. We used a well-characterized rat model of IUGR to test these hypotheses (5, 29–31, 44).
MATERIALS AND METHODS
Animals.
The rat UPI model of IUGR has been described in detail previously (27, 28, 59). All procedures were approved by the University of Utah Animal Care Committee and are in accordance with the American Physiological Society's guiding principles (1). Body weights of IUGR pups are ∼25% smaller than the control pups (22). The surgical procedures have been described previously (26, 45). Briefly, on day 19 of gestation, pregnant Sprague-Dawley rats were anesthetized with intraperitoneal xylazine (8 mg/kg) and ketamine (40 mg/kg), and both uterine arteries were ligated giving rise to IUGR pups. Control dams underwent identical anesthetic procedures. After recovery, rats were given ad libitum access to food and water.
Day 0 (d0) pups were delivered by caesarian section at term, 2.5 days after bilateral uterine artery ligation. For day 7 (d7) and day 21 (d21) pups, dams were allowed to deliver spontaneously and litters randomly culled to six pups. Pups were raised to d21 by their own dams. IUGR pups were not cross-fostered as we have previously demonstrated that maternal rat milk from dams that that have undergone IUGR surgery does not significantly differ from control dam milk in terms of volume, calories, fat, protein, zinc, and sodium content (24). For all ages, lungs were removed upon killing, flash-frozen, and stored at −80°C or insufflated via the trachea with 10% buffered formalin at 20 cmH2O. Molecular experiments used tissue from 12 pups (six male and six female from each group), immunohistochemistry used five pups (two or three male and two or three female). Parallel studies were done to measure static lung compliance using five pups in both the control and IUGR groups (two or three male and two or three female). To ensure litter-litter variation, pups for each experiment were randomly selected from different litters. For mRNA experiments, one male and one female pup were randomly selected from each litter; for IHC and compliance experiments, one pup (male or female) was randomly selected from each litter.
IUGR pups weigh ∼25% less than controls at d0, 20% less than controls at d7, and 15% less than controls at d21 (22). At birth, there is no significant difference in lung-body weight ratios between IUGR and control pups (43).
Real-time RT PCR.
Real-time reverse transcriptase PCR was used to evaluate mRNA abundance of elastin, as well as mRNA of genes that regulate synthesis and assembly of elastin fibers: including TGF-α and -β1, fibrillin-1, fibulin-1, and lysyl oxidase and were performed as previously described (22). The following assay-on-demand primer/probe sets were used: elastin, Rn01299782_ml; TGF-α, Rn00446234_m1; TGF-β, Rn99999016_ml; fibrillin-1, Rn00582774_m1; fibulin-1, Rn01504529_m1; and lysyl oxidase, Rn00566984_m1 (Applied Biosystems). Levels of mRNA were determined using the comparative Ct method (33) with GAPDH as an internal control (GAPDH primer and probe sequences; forward, CAAGATGGTGAAGGTCGGTGT; reverse, CAAGAGAAGGCAGCCCTGGT; probe, GCGTCCGATACGGCCAAATCCG).
Quantitative elastin histology.
Tissue was embedded in paraffin, and 5 μm sections were cut. Sections were stained with Hart's elastic fiber stain to assess elastic fibers in lung parenchyma. A systematic sampling method was used to evaluate random, nonoverlapping calibrated fields. The Bioquant True Color Windows Image Analysis system (R & M Biometrics, Nashville, TN) was used to make measurements (2, 8, 19). Elastic fiber density was quantified as previously described (7). Briefly, color thresholds were set for elastic fibers (stained purple-black) and nonelastic tissue (stained yellow) in 15 random, calibrated areas of lung parenchyma/section (1 section/rat lung). The calibrated pixel area for elastin was divided by the calibrated pixel area for nonelastic tissue to calculate the percent area occupied by elastic fibers. Airways and large vessels were excluded from analysis.
Elastase activity.
Elastase activity was measured in d21 lungs using EnzChek Elastase Assay Kit (E-12056, Molecular Probes), in the presence of the elastase inhibitor N-methoxysuccinyl-Ala-Ala-Val-chloromethyl ketone, according to manufacturer's instructions.
Static compliance measurements.
Static compliance was calculated for d21 rat pups from deflation pressure-volume curves. Compliance experiments were not performed on d0 or d7 pups due to their small size. Rat pups were anesthetized with xylazine (8 mg/kg) and ketamine (10 mg/kg), and pancuronium (10 mg/kg) was administered to facilitate ventilation. All rats underwent tracheostomy and were ventilated with a Bird VIP Ventilator using the following settings (FiO2 = 100%, I:E = 0.33, RR = 60/min, flow = 31/min, PIP = 12 cmH2O, PEEP = 2 cmH2O). When oxygen saturations were stable at >97%, rats were quickly disconnected from the ventilator, and lungs were inflated to total lung capacity with 2.5 ml of air. The chest was closed. The resulting static airway pressure was measured at zero flow. Sequential volumes of 3.0, 2.5, 2.0, 1.5, and 1.0 ml of volume were delivered, with repeated measure of airway pressure. Rats were returned to the ventilator, stabilized, and the procedure repeated three times for each rat.
Airway pressure and flow were acquired as analog signals, using a Hugo-Sachs differential pressure transducer and a hot film anemometer, respectively. The analog signals were digitized using a National Instruments USB-6211. Labview software was used to acquire the pressure waveform during the delivery of 2.5, 2.0, 1.5, 1.0, and 0.5 ml volumes of air. Deflation measurements were made from highest to lowest volumes to minimize recruitment artifacts. Static compliance was calculated from the slope of pressure-volume curves between of 1 and 2 ml. We chose to calculate static lung compliance at these lower volumes to examine elastic recoil forces. The most significant contribution of elastin to compliance will occur at volumes slightly greater than functional residual capacity, which in the rat lung, at d21, is ∼1 ml (9, 51).
Statistics.
Data are presented as means ± standard deviation (SD), unless otherwise noted. Statistical significance was determined using nonparametric Mann-Whitney test, using the Statview software package (SAS Institute, Cary, NC). P ≤ 0.05 was considered significant.
RESULTS
IUGR decreases rat lung elastin mRNA levels at birth and d7 but not at d21.
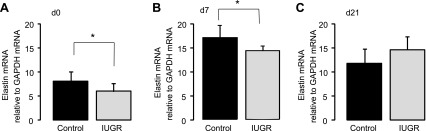
The effect of IUGR on elastin mRNA levels in rat lungs was evaluated by real-time reverse transcriptase PCR. Transcript levels of elastin mRNA were measured relative to GAPDH in control and IUGR whole lung at d0, d7, and d21 (n = 12/day). IUGR decreased elastin mRNA transcript levels compared with age-matched control at d0 (P = 0.01) and at d7 (P = 0.02) (Fig. 1, A and B). At d21, IUGR did not significantly alter elastin mRNA transcript levels (Fig. 1C). Data were also analyzed for sex-specific changes in elastin mRNA, and none were observed.
Fig. 1.
Elastin mRNA levels. Intrauterine growth restriction (IUGR) decreases elastin mRNA levels in neonatal rat lung at postnatal day (d) 0 (A) and d7 (B) but not at d21 (C). Bars are means ± SD of 12 rats (6 male and 6 female). *P ≤ 0.01.
IUGR decreases rat lung elastic fiber levels at d21.
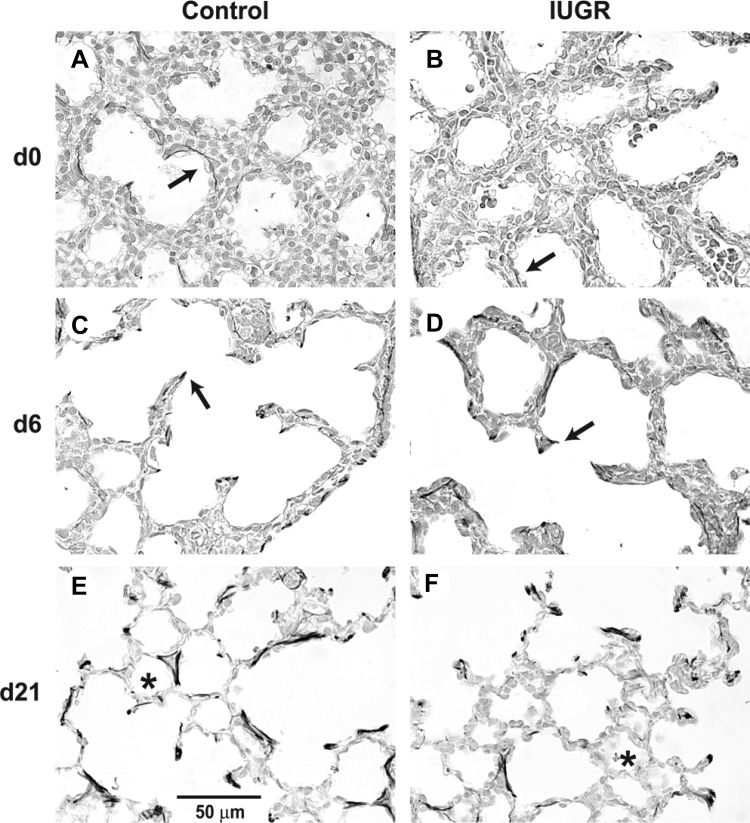
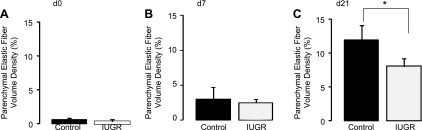
Elastic fiber abundance in alveolar walls of control and IUGR lungs was revealed by Hart's elastic fiber stain. Elastic fibers were detected at the tips of alveolar septa, within developing alveolar walls and around blood vessels (airways and large vessels were not included in quantification) (Fig. 2). Quantitative analysis of elastic fiber density demonstrated that IUGR did not significantly alter elastic fiber accumulation in the lung parenchyma in d0 (P = 0.56) or d7 rats (0.79) (Fig. 3, A and B). However, in the lung of d21 rats, IUGR significantly decreased elastic fiber accumulation in the lung parenchyma (P = 0.0023) (Fig. 3C).
Fig. 2.
Hart's stained lung tissue. Images show elastic fibers (black stain, arrows) in d0 (A and B), d7 (C and D) and d21 (E and F) lungs of control vs. IUGR rat pups. Elastic fiber deposition in parenchymal walls appears less in the IUGR rat pup at d21 compared with the matched control (*).
Fig. 3.
Quantitative assessment of Hart's stain. IUGR does not alter elastic fiber density in IUGR lungs at d0 (A) or d7 (B). IUGR decreases parenchymal elastic fiber density in mature rat lung (C) at d21. Bars are means ± SD of 5 rats (mixed male and female). *P ≤ 0.01.
IUGR does not alter lung elastase activity at d21.
IUGR did not significantly alter lung elastase activity in d21 rat lungs (control 0.022 ± 0.01 U/mg protein, IUGR 0.026 ± 0.006 U/mg protein).
IUGR decreases rat lung mRNA of genes essential for elastic fiber formation at birth and d7, but not at d21.
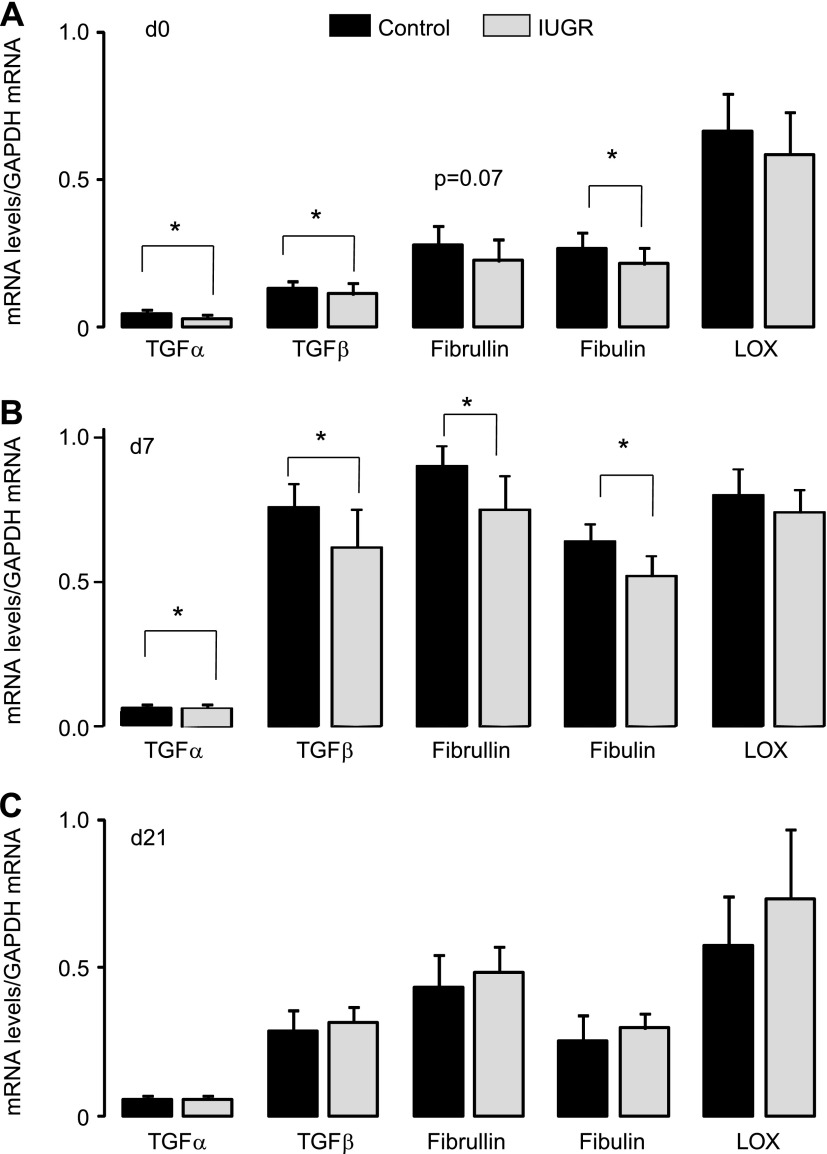
The effect of IUGR on mRNA levels of genes that regulate synthesis and assembly of elastin fibers was determined using real-time RT-PCR. Transcript levels of TGF-α and -β, fibrillin-1, fibulin-1, and lysyl oxidase mRNA were quantified relative to GAPDH in control and IUGR whole lung tissue homogenates at d0, d7, and d21 (n = 12/d). At d0, IUGR significantly decreased mRNA levels of TGF-α (P = 0.003), TGF-β (P = 0.04), and fibulin-1 (P = 0.03) compared with control (Fig. 4A). At d7, IUGR significantly decreased mRNA levels of TGF-α (P = 0.02), TGF-β (P = 0.02), fibrillin (0.03), and fibulin-1 (P = 0.002) compared with control (Fig. 4B). At d21, IUGR did not significantly alter rat lung mRNA levels of TGF-α, TGF-β, fibrillin-1, fibulin-1, or lysyl oxidase (Fig. 4C).
Fig. 4.
mRNA levels of genes involved in elastin deposition and synthesis. IUGR decreases transforming growth factor (TGF)-α, TGF-β, fibrillin, and fibulin mRNA levels in neonatal rat lung at d0 (A) and d7 (B) but not at d21 (C). Bars are means ± SD of 12 rats (6 male and 6 female). *P ≤ 0.01.
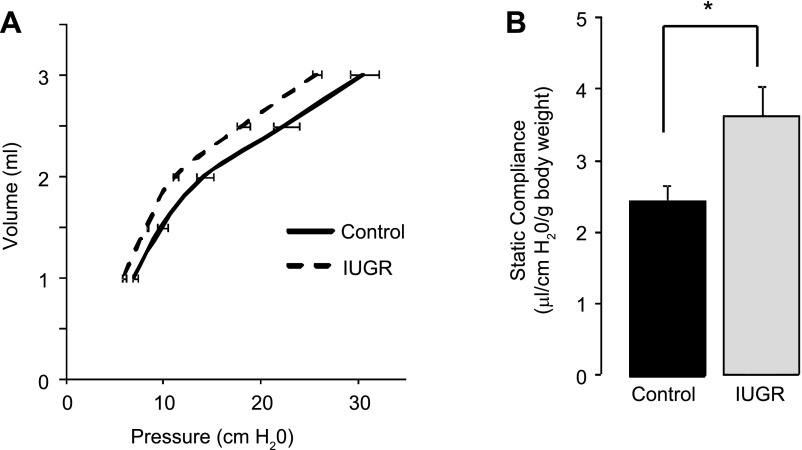
IUGR increases static lung compliance in d21 rat lung.
Deflation pressure volume curves were used to calculate static lung compliance in control and IUGR rats at d21. At all lung volumes, the IUGR pressure-volume curve is shifted to the left relative to controls (Fig. 5A). IUGR increased static lung compliance (P = 0.009) in d21 rats compared with age-matched controls (Fig. 5B).
Fig. 5.
Pressure-volume curves and static lung compliance. IUGR increases closed chest, static compliance in d21 rat lungs. A: pressure volume curves of d21 Control and IUGR rats. B: control and IUGR static compliance in d21 rat lung/g body wt. Bars are means ± SD of 5 rats (mixed male and female).*P ≤ 0.001.
DISCUSSION
In this study, we demonstrated that UPI-induced IUGR decreases mRNA transcript levels of elastic fiber synthesis genes, before alveolarization (d0), and during alveolarization (d7) in the rat. The decrease in elastic fiber synthesis genes is associated with a reduced elastic fiber density in the developmentally mature IUGR lung (d21) and an increase in static lung compliance. We conclude that IUGR decreased lung elastin deposition in the rat. These findings are novel and imply that the mechanism by which IUGR predisposes to pulmonary disease may be via decreased lung elastic fiber deposition.
Normal deposition and arrangement of elastic fibers is important in alveolar formation and the prevention of alveolar degeneration, such as that seen in emphysema and chronic obstructive pulmonary disease (6, 37, 39, 51, 61). Elastin deposition, in turn, is dependent upon timely and robust expression of elastin and accompanying factors that collectively function to assemble elastic fibers (37). Lung elastin expression is maximal during alveolar formation, which in rats occurs during postnatal days ∼4–14 (37). The regulation of elastin synthesis and deposition through its peak production during lung development is transcriptionally mediated. Later in life, after alveolarization is complete, the regulation of elastin synthesis and deposition becomes posttranscriptional (56). Importantly, the IUGR insult may only affect the transcriptional regulation of elastin and therefore only alter elastin mRNA transcript levels prior to, and during, alveolarization. A reduction in elastin mRNA at d0 and d7 may render a less abundant supply of elastin for deposition during alveolar formation. This is consistent with our findings of a reduced elastic fiber density in the d21 rat lung.
Elastic fiber formation also depends upon the coordinated expression of number of other genes that regulate the induction and assembly of elastin fibers. Dysregulated elastin production and fiber formation is positively associated with changes in mRNA levels of genes including, TGF-α, TGF-β, fibrillin, fibulin, and lysyl oxidase (7, 50, 55). Our observed decrease in TGF-α, TGF-β, fibrillin, and fibulin mRNA transcript levels in the d0 and d7 rat lung is consistent with a reduced ability to deposit elastin during alveolarization and likely also contributes to the reduced elastic fiber deposition the mature (d21) lung.
Another modulator of elastin expression in the mouse is the transcription factor PPARγ (52, 54). The PPARγ-targeted lung epithelial total knockout mouse has decreased expression of lung extracellular matrix genes, including elastin, in response to epithelial PPARγ deletion (53). At 8–12 wk of age, these mice also have more compliant lungs with reduced radial alveolar counts (54). In addition to having decreased elastin (10, 12), neonatal rat pups exposed to hyperoxia, also have decreased PPARγ expression and impaired alveolarization, which is reversed with the synthetic PPARγ agonist, rosiglitazone (47). Notably, we have previously demonstrated that UPI-induced IUGR decreases PPARγ1 and -γ2 protein levels, as well as downstream chromatin modifying enzyme Setd8, in the rat lung at birth (20). This is important because changes in chromatin-modifying enzymes, such as Setd8, have the potential to change the epigenetic regulation of expression of targeted genes. It will be necessary to determine the extent to which PPARγ-induced changes in Setd8 directly affect elastin expression in the IUGR rat lung.
Lung elastin content in the heterozygotic Eln+/− mouse is ∼50% of the wild-type mouse, a reduction similar in magnitude to what we have observed in the IUGR rat lung at d21 (51). In the Eln+/− mouse, this modest reduction in elastin is associated with an increase in static lung compliance (51). Our observation of increased static lung compliance in the IUGR rat is consistent with this. We speculate that the increased static lung compliance we observed is a result of reduced lung elastic fiber deposition in the d21 lung. A significant implication of these findings is that, as in the Eln+/− mouse, reduced elastic fiber deposition may render the mature IUGR lung more susceptible to lung injury after an additional postnatal insult.
A number of postnatal lung insults including mechanical ventilation (MV) and hyperoxia are associated with increased lung elastic fiber deposition. Preterm infants with BPD, who are oxygenated with MV and/or received supplemental oxygen, have increased elastin associated with reduced septation and fewer alveoli (35, 57). Preterm lambs subject to MV display disordered and excessive elastin production and dysregulated expression of genes whose protein products are involved in elastin fiber formation (3, 7). It is possible that reduced elastic fiber deposition in the lung of IUGR infants, particularly those who are also preterm, may the stage for an exaggerated repair response when faced with a secondary insult.
A significant limitation of this study is the measurement of static compliance under closed chest conditions. Chest wall stiffness may vary between control and IUGR rats. Differences in chest wall stiffness would affect compliance and have not been assessed in this study. A further limitation is the use of a rat model. While rat lung development follows a similar course to human lung development, it is important to remain cognizant of the fundamental differences. For example, the rat lung is adequately functional when in the saccular stage of development, as opposed to the human lung at the same stage of development. An advantage of using a rat model to study lung development and the effects IUGR on lung development, however, is that the immature lung can be interrogated, without the complications of prematurity. In this study, we have not assessed the elastin content of the IUGR lung in older rats. It will be important to determine if the reduction in lung elastin observed in the d21 IUGR rat persists in the mature animal. As such, future studies examining elastin content and lung compliance in adult IUGR rats will be necessary.
In conclusion, the lung of IUGR rat pups appears to be poised for the development of disease. Specifically, at birth and d7, IUGR decreases elastin mRNA transcript levels, before the completion of alveolarization. At d21, when alveolarization is complete, the IUGR lung is characterized by decreased elastic fiber deposition and increased static lung compliance. These findings are novel and suggest that lung elastin deposition is a mechanism by which IUGR predisposes to later onset lung disease.
GRANTS
This work was supported by the National Institutes of Health, University of Utah's Children's Health Research Center, and the Primary Children's Medical Center Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We wish to acknowledge the support of J. Ross Milley, Division of Neonatology.
REFERENCES
- 1. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. [DOI] [PubMed] [Google Scholar]
- 2. Albertine KH, Dahl MJ, Gonzales LW, Wang ZM, Metcalfe D, Hyde DM, Plopper CG, Starcher BC, Carlton DP, Bland RD.Chronic lung disease in preterm lambs: effect of daily vitamin A treatment on alveolarization. Am J Physiol Lung Cell Mol Physiol 299: L59–L72, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, Carlton DP, Bland RD.Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med 159: 945–958, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Aucott SW, Donohue PK, Northington FJ.Increased morbidity in severe early intrauterine growth restriction. J Perinatol 24: 435–440, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Baserga M, Hale MA, McKnight RA, Yu X, Callaway CW, Lane RH.Uteroplacental insufficiency alters hepatic expression, phosphorylation, and activity of the glucocorticoid receptor in fetal IUGR rats. Am J Physiol Regul Integr Comp Physiol 289: R1348–R1353, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Black PN, Ching PS, Beaumont B, Ranasinghe S, Taylor G, Merrilees MJ.Changes in elastic fibres in the small airways and alveoli in COPD. Eur Respir J 31: 998–1004, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, Fong KS.Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 292: L1370–L1384, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Bolender RP, Hyde DM, Dehoff RT.Lung morphometry: a new generation of tools and experiments for organ, tissue, cell, and molecular biology. Am J Physiol Lung Cell Mol Physiol 265: L521–L548, 1993. [DOI] [PubMed] [Google Scholar]
- 9. Bolle I, Eder G, Takenaka S, Ganguly K, Karrasch S, Zeller C, Neuner M, Kreyling WG, Tsuda A, Schulz H.Postnatal lung function in the developing rat. J Appl Physiol 104: 1167–1176, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Bruce MC, Bruce EN, Janiga K, Chetty A.Hyperoxic exposure of developing rat lung decreases tropoelastin mRNA levels that rebound postexposure. Am J Physiol Lung Cell Mol Physiol 265: L293–L300, 1993. [DOI] [PubMed] [Google Scholar]
- 11. Bruce MC, Lo PY.A morphometric quantitation of developmental changes in elastic fibers in rat lung parenchyma: variability with lung region and postnatal age. J Lab Clin Med 117: 226–233, 1991. [PubMed] [Google Scholar]
- 12. Bruce MC, Pawlowski R, Tomashefski JF., JrChanges in lung elastic fiber structure and concentration associated with hyperoxic exposure in the developing rat lung. Am Rev Respir Dis 140: 1067–1074, 1989. [DOI] [PubMed] [Google Scholar]
- 13. Cetin I, Alvino G.Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 30, Suppl A: S77–82, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Frusca T, Soregaroli M, Valcamonico A, Guandalini F, Danti L.Doppler velocimetry of the uterine arteries in nulliparous women. Early Hum Dev 48: 177–185, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Greenough A, Yuksel B, Cheeseman P.Effect of in utero growth retardation on lung function at follow-up of prematurely born infants. Eur Respir J 24: 731–733, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Harding R, Cock ML, Louey S, Joyce BJ, Davey MG, Albuquerque CA, Hooper SB, Maritz GS.The compromised intra-uterine environment: implications for future lung health. Clin Exp Pharmacol Physiol 27: 965–974, 2000. [DOI] [PubMed] [Google Scholar]
- 17. Hoo AF, Stocks J, Lum S, Wade AM, Castle RA, Costeloe KL, Dezateux C.Development of lung function in early life: influence of birth weight in infants of nonsmokers. Am J Respir Crit Care Med 170: 527–533, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B.Annual summary of vital statistics: 2004. Pediatrics 117: 168–183, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Hsia CC, Hyde DM, Ochs M, Weibel ER.An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joss-Moore LA, Wang Y, Baack ML, Yao J, Norris AW, Yu X, Callaway CW, McKnight RA, Albertine KH, Lane RH.IUGR decreases PPARg and Setd8 expression in neonatal rat lung and these effects are ameliorated by maternal DHA supplementation. Early Hum Dev 86: 785–791, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joss-Moore LA, Wang Y, Campbell MS, Moore B, Yu X, Callaway CW, McKnight RA, Desai M, Moyer-Mileur LJ, Lane RH.Uteroplacental insufficiency increases visceral adiposity and visceral adipose PPARgamma2 expression in male rat offspring prior to the onset of obesity. Early Hum Dev 86: 179–185, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karadag A, Sakurai R, Wang Y, Guo P, Desai M, Ross MG, Torday JS, Rehan VK.Effect of maternal food restriction on fetal rat lung lipid differentiation program. Pediatr Pulmonol 44: 635–644, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, Yu X, Wang L, Callaway CW, Gill G, Chan GM, Albertine KH, McKnight RA, Lane RH.Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics 25: 16–28, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Kinzler WL, Vintzileos AM.Fetal growth restriction: a modern approach. Curr Opin Obstet Gynecol 20: 125–131, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Kloesz JL, Serdikoff CM, Maclennan NK, Adibi SA, Lane RH.Uteroplacental insufficiency alters liver and skeletal muscle branched-chain amino acid metabolism in intrauterine growth-restricted fetal rats. Pediatr Res 50: 604–610, 2001. [DOI] [PubMed] [Google Scholar]
- 27. Lane RH, Chandorkar AK, Flozak AS, Simmons RA.Intrauterine growth retardation alters mitochondrial gene expression and function in fetal and juvenile rat skeletal muscle. Pediatr Res 43: 563–570, 1998. [DOI] [PubMed] [Google Scholar]
- 28. Lane RH, Dvorak B, MacLennan NK, Dvorakova K, Halpern MD, Pham TD, Philipps AF.IGF alters jejunal glucose transporter expression and serum glucose levels in immature rats. Am J Physiol Regul Integr Comp Physiol 283: R1450–R1460, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Lane RH, Flozak AS, Ogata ES, Bell GI, Simmons RA.Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr Res 39: 390–394, 1996. [DOI] [PubMed] [Google Scholar]
- 30. Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD.Increased hepatic peroxisome proliferator-activated receptor-gamma coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology 143: 2486–2490, 2002. [DOI] [PubMed] [Google Scholar]
- 31. Lane RH, Tsirka AE, Gruetzmacher EM.Uteroplacental insufficiency alters cerebral mitochondrial gene expression and DNA in fetal and juvenile rats. Pediatr Res 47: 792–797, 2000. [DOI] [PubMed] [Google Scholar]
- 32. Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C.Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124: 3943–3953, 1997. [DOI] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD.Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 34. Lucas JS, Inskip HM, Godfrey KM, Foreman CT, Warner JO, Gregson RK, Clough JB.Small size at birth and greater postnatal weight gain: relationships to diminished infant lung function. Am J Respir Crit Care Med 170: 534–540, 2004. [DOI] [PubMed] [Google Scholar]
- 35. Margraf LR, Tomashefski JF, Jr, Bruce MC, Dahms BB.Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis 143: 391–400, 1991. [DOI] [PubMed] [Google Scholar]
- 36. Mariani TJ, Dunsmore SE, Li Q, Ye X, Pierce RA.Regulation of lung fibroblast tropoelastin expression by alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 274: L47–L57, 1998. [DOI] [PubMed] [Google Scholar]
- 37. Mariani TJ, Sandefur S, Pierce RA.Elastin in lung development. Exp Lung Res 23: 131–145, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Maritz GS, Cock ML, Louey S, Joyce BJ, Albuquerque CA, Harding R.Effects of fetal growth restriction on lung development before and after birth: a morphometric analysis. Pediatr Pulmonol 32: 201–210, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Maritz GS, Cock ML, Louey S, Suzuki K, Harding R.Fetal growth restriction has long-term effects on postnatal lung structure in sheep. Pediatr Res 55: 287–295, 2004. [DOI] [PubMed] [Google Scholar]
- 40. McGowan SE, McNamer R.Transforming growth factor-beta increases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol 3: 369–376, 1990. [DOI] [PubMed] [Google Scholar]
- 41. McIntire DD, Bloom SL, Casey BM, Leveno KJ.Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 340: 1234–1238, 1999. [DOI] [PubMed] [Google Scholar]
- 42. Minior VK, Divon MY.Fetal growth restriction at term: myth or reality? Obstet Gynecol 92: 57–60, 1998. [DOI] [PubMed] [Google Scholar]
- 43. O'Brien EA, Barnes V, Zhao L, McKnight RA, Yu X, Callaway CW, Wang L, Sun JC, Dahl MJ, Wint A, Wang Z, McIntyre TM, Albertine KH, Lane RH.Uteroplacental insufficiency decreases p53 serine-15 phosphorylation in term IUGR rat lungs. Am J Physiol Regul Integr Comp Physiol 293: R314–R322, 2007. [DOI] [PubMed] [Google Scholar]
- 44. Ogata ES, Bussey ME, Finley S.Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism 35: 970–977, 1986. [DOI] [PubMed] [Google Scholar]
- 45. Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH.Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol 285: R962–R970, 2003. [DOI] [PubMed] [Google Scholar]
- 46. Regev RH, Lusky A, Dolfin T, Litmanovitz I, Arnon S, Reichman B.Excess mortality and morbidity among small-for-gestational-age premature infants: a population-based study. J Pediatr 143: 186–191, 2003. [DOI] [PubMed] [Google Scholar]
- 47. Rehan VK, Wang Y, Patel S, Santos J, Torday JS.Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol 41: 558–569, 2006. [DOI] [PubMed] [Google Scholar]
- 48. Reiss I, Landmann E, Heckmann M, Misselwitz B, Gortner L.Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch Gynecol Obstet 269: 40–44, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Rosenberg A.The IUGR newborn. Semin Perinatol 32: 219–224, 2008. [DOI] [PubMed] [Google Scholar]
- 50. Saha A, Wittmeyer J, Cairns BR.Chromatin remodelling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol 7: 437–447, 2006. [DOI] [PubMed] [Google Scholar]
- 51. Shifren A, Durmowicz AG, Knutsen RH, Hirano E, Mecham RP.Elastin protein levels are a vital modifier affecting normal lung development and susceptibility to emphysema. Am J Physiol Lung Cell Mol Physiol 292: L778–L787, 2007. [DOI] [PubMed] [Google Scholar]
- 52. Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Andalcio T, Shapiro SD, Mariani TJ.Epithelial cell PPARgamma is an endogenous regulator of normal lung maturation and maintenance. Proc Am Thorac Soc 3: 510–511, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, Gonzalez F, Shapiro SD, Mariani TJ.Epithelial cell PPARgamma contributes to normal lung maturation. FASEB J 20: 1507–1509, 2006. [DOI] [PubMed] [Google Scholar]
- 54. Simon DM, Tsai LW, Ingenito EP, Starcher BC, Mariani TJ.PPAR gamma deficiency results in reduced lung elastic recoil and abnormalities in airspace distribution. Respir Res 11: 69, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Srisuma S, Bhattacharya S, Simon DM, Solleti SK, Tyagi S, Starcher B, Mariani TJ.Fibroblast growth factor receptors control epithelial-mesenchymal interactions necessary for alveolar elastogenesis. Am J Respir Crit Care Med 181: 838–850, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Swee MH, Parks WC, Pierce RA.Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J Biol Chem 270: 14899–14906, 1995. [DOI] [PubMed] [Google Scholar]
- 57. Thibeault DW, Mabry SM, Ekekezie II, Truog WE.Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 106: 1452–1459, 2000. [DOI] [PubMed] [Google Scholar]
- 58. Tyson JE, Kennedy K, Broyles S, Rosenfeld CR.The small for gestational age infant: accelerated or delayed pulmonary maturation? Increased or decreased survival? Pediatrics 95: 534–538, 1995. [PubMed] [Google Scholar]
- 59. Unterman TG, Simmons RA, Glick RP, Ogata ES.Circulating levels of insulin, insulin-like growth factor-I (IGF-I), IGF-II, and IGF-binding proteins in the small for gestational age fetal rat. Endocrinology 132: 327–336, 1993. [DOI] [PubMed] [Google Scholar]
- 60. Wagenseil JE, Mecham RP.New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 81: 229–240, 2007. [DOI] [PubMed] [Google Scholar]
- 61. Warburton D, Gauldie J, Bellusci S, Shi W.Lung development and susceptibility to chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 668–672, 2006. [DOI] [PubMed] [Google Scholar]
- 62. Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY.Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23: 320–326, 2000. [DOI] [PubMed] [Google Scholar]
- 63. Zimmermann P, Eirio V, Koskinen J, Kujansuu E, Ranta T.Doppler assessment of the uterine and uteroplacental circulation in the second trimester in pregnancies at high risk for pre-eclampsia and/or intrauterine growth retardation: comparison and correlation between different Doppler parameters. Ultrasound Obstet Gynecol 9: 330–338, 1997. [DOI] [PubMed] [Google Scholar]