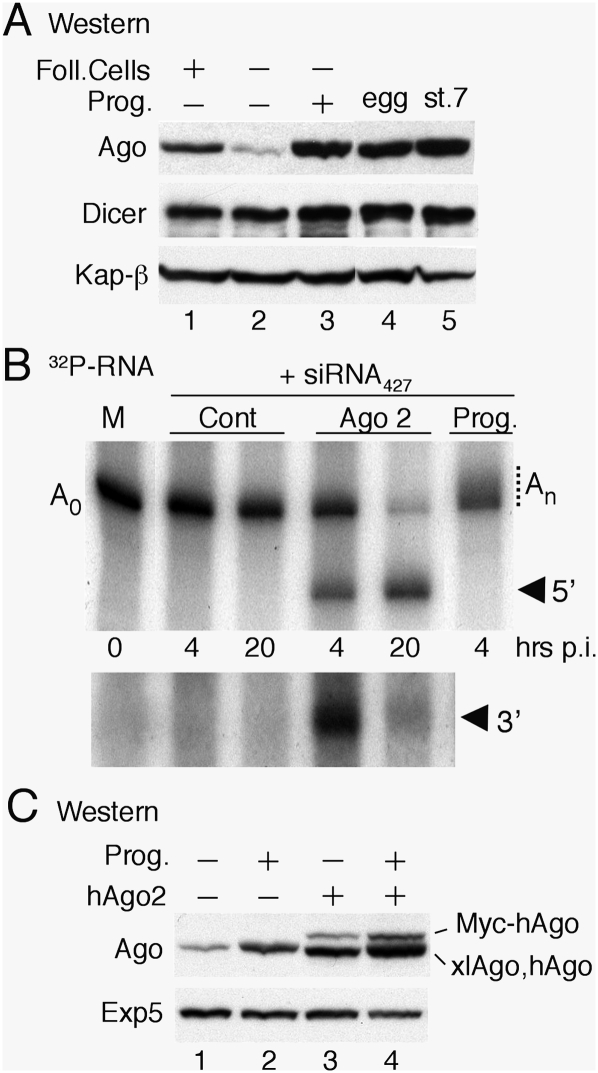
Figure 6.
Absence of Ago2 catalytic activity from Xenopus oocytes. (A) Up-regulation of Ago protein levels during oocyte maturation. Whole-cell extracts from oocytes (lanes 1–3), eggs (lane 4), and stage 7 embryos (lane 5) were analyzed by Western blotting as in Figure 3. Immature (stage VI) oocytes were untreated (lane 1) or treated to remove follicle cells (lane 2) and matured by treatment with progesterone (lane 3). (B) Lack of RNAi in oocytes in the absence of exogenous Ago2. A 32P-labeled reporter RNA containing a perfect match miR-427 target site in the 3′ UTR (cf. Fig. 4) was coinjected with siRNA427 (20 fmol per embryo) into the cytoplasms of control oocytes (Cont), oocytes preinjected with synthetic mRNA encoding Myc-tagged hAgo2 (Ago2), or matured oocytes (Prog.), which supported polyadenylation (An). The bottom panel shows a longer autoradiographic exposure for detection of the shorter 3′ cleavage product. (C) Normal up-regulation of endogenous xlAgo proteins in the presence of exogenous hAgo2. Accumulation of Ago proteins in immature oocytes (lanes 1,3) or matured oocytes (lanes 2,4) in the absence (lanes 1,2) or presence of exogenous hAgo2 (lanes 3,4) was monitored as in A.