Figure 1.
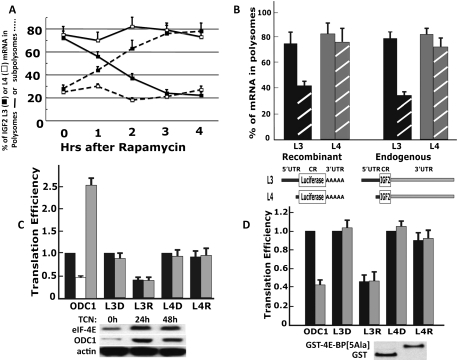
The rapamycin-sensitive translational initiation of IGF2 L3 mRNA is mediated by its 5′ UTR and is independent of eIF-4E. (A) The effect of rapamycin on the polysomal association of IGF2 L3 and L4 mRNAs in rapidly growing RD cells. Rapamycin (20 nM) was added to RD cells at ∼30% confluency; the cells were harvested at intervals thereafter and extracts were subjected to sucrose density gradient centrifugation. The subpolysomal ([- - -] fractions 1–5, Supplemental Fig. S2) and polysomal ([—] fractions 6–13) regions of the gradient were pooled separately, and each was assayed by qPCR for the content of IGF2 L3 (▪) and L4 (□) mRNAs. The combined results of three experiments are shown ±SEM. (B) The rapamycin sensitivity of IGF2 L3 mRNA translational initiation is conferred by the L3 5′ UTR. The IGF2 L3 and L4 5′ UTR segments were fused to the coding sequences of firefly luciferase (see cartoon) and were transiently transfected into rapidly growing RD cells. After 24 h, cells were treated either with DMSO (filled bars) or rapamycin (20 nM, 3 h.; cross-hatched bars); extracts were separated by sucrose density gradient centrifugation, and the content of endogenous IGF2 L3 (black-filled and hatched bars, right) and L4 (gray-filled and hatched bars, right) mRNAs and of the L3-luciferase (black-filled and hatched bars, left) and L4-luciferase (gray-filled and hatched bars, left) mRNAs in the pooled polysomal fractions was determined by qPCR and is expressed as a percentage of the corresponding total L3 or L4 mRNA in the sample loaded. The combined results of three experiments are shown ±SEM. (C) Overexpression of eIF-4E up-regulates ODC1-luciferase expression but does not alter the expression of L3-luciferase. Plasmids encoding the 5′ UTR of human ODC1 fused to firefly luciferase or L3-firefly luciferase or L4-firefly luciferase, each together with a plasmid encoding Renilla luciferase, were transfected into RD cells that stably express recombinant eIF-4E in a tetracycline-inducible manner. The cells were treated with tetracycline (gray bars) or carrier (black bars) for 24 h. Three hours prior to harvest, cells were treated with DMSO (D) or rapamycin (20 nM, 3 h; R). Thereafter, extracts were assayed for firefly and Renilla luciferase activity and by qPCR for the content of firefly luciferase mRNA. The activity of firefly luciferase was divided by the activity of Renilla luciferase to give a normalized firefly luciferase activity; “translational efficiency” was calculated by dividing the normalized firefly luciferase activity by the measured content of firefly luciferase mRNA, setting to 1 the value of this dividend for the ODC1-luciferase, L3-luciferase, and L4-luciferase conditions in the absence of tetracycline induction or rapamycin treatment (the black bars in ODC1, L3D, and L4D). The unfilled/white bar shows the effect of rapamycin on the expression of ODC1-luciferase in the absence of tetracycline. Each experiment was performed in triplicate, and the combined results of three experiments are shown ±SEM. (D) Overexpression of a nonphosphorylatable mutant of 4E-BP1 suppresses expression of ODC1-luciferase but does not alter expression of L3-luciferase. RD cells were engineered to stably overexpress GST or a GST fused to a nonphosphorylatable 4E-BP1 polypeptide (GST-4E-BP[5Ala]) (Hara et al. 2002). Plasmids encoding ODC1-luciferase, L3-luciferase, or L4-luciferase, each together with a plasmid encoding Renilla luciferase, were transfected into the RD GST (black bars) or GST-4E-BP[5Ala] (gray bars) stable transformants. After 24 h, the cells were treated with DMSO (D) or rapamycin (20 nM, 3 h; R). Translational efficiency was calculated as in C, and set to 1 for the ODC1-luciferase, L3-luciferase, and L4-luciferase conditions in the extracts from cells expressing GST in the absence of rapamycin treatment (the black bars in ODC1, L3D, and L4D). Each experiment was performed in triplicate, and the combined results of three experiments are shown ±SEM.