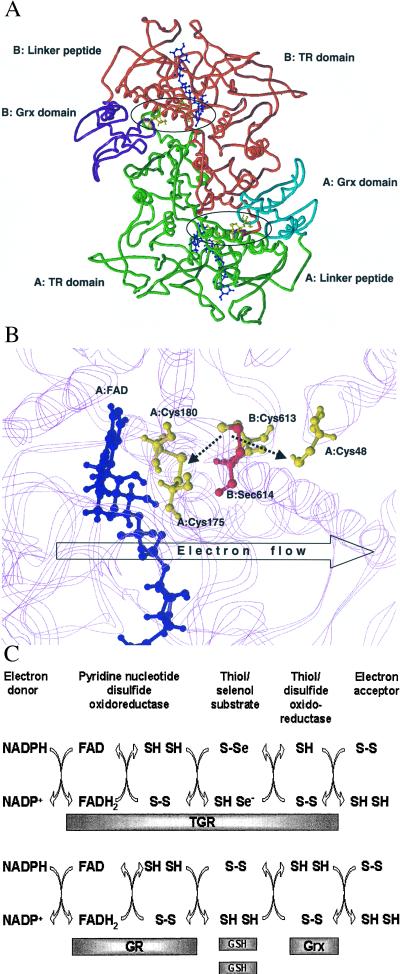
Figure 4.
Molecular modeling and reaction mechanism of TGR. (A) Molecular model of the TGR homodimer. The three-dimensional model was obtained as described under Experimental Procedures. The pyridine nucleotide disulfide oxidoreductase portion and the C-terminal extension (TR domain) of subunit A are shown in green and of subunit B in red. The Grx domain of subunit A is shown in cyan and of subunit B in purple. The location of the TR and Grx domains and the peptide that links these domains (linker peptide) are indicated for both subunits. Active centers are circled. Two FAD molecules are shown in blue, and Cys-48, Cys-175, Cys-180, Cys-613, and Sec-614 in both subunits are shown in yellow. (B) Molecular model of the enzyme active center. FAD molecule is shown in blue. Cys-48, Cys-175, and Cys-180 in subunit A and Cys-613 in subunit B are shown in yellow. Sec-614 in subunit B is shown in red. The location of protein chains that surround the active center are shown in the background in magenta. The Cys-613/Sec-614 and Cys-175/Cys-180 pairs are shown in the oxidized state. Predicted interactions of the Cys-613/Sec-614 dipeptide with the disulfide center (Cys-175/Cys-180) and the active center of the Grx domain (Cys-48) are shown by dashed arrows. In the initial step of catalysis by TGR, NADPH reduces FAD. The predicted direction of electron flow in subsequent steps of catalysis (A: FAD→A: Cys-175/Cys-180→B: Cys-613/Sec-614→A: Cys-48) is shown by the open arrow. (C) Reaction mechanism of TGR. Proposed electron flow involving TGR (Upper) and, for comparison, components of the GSH system (Lower) is shown. TGR is functionally and structurally analogous to a fusion of GR, Grx, and two molecules of GSH and supports five electron transfer events. The roles of redox groups are as follows: the thiol/disulfide redox center initially accepts electrons from NADPH via FAD. In the GSH system, GSSG is then directly reduced by the thiol/disulfide center, whereas in TGR, the C-terminal tetrapeptide serves a role of a protein-linked GSSG and is reduced by the thiol/disulfide center. Electrons are further transferred from GSH to Grx in the GSH system or by a conformational movement of the C-terminal tetrapeptide of TGR from the thiol/disulfide center to the Grx domain. Finally, Grx or the Grx domain interact with an electron acceptor (for example, GSH-mixed disulfide), completing the reaction.