FIG. 2. In vitro analysis of A-PG formation (A), modified in vitro analysis of A-PGS catalysis using different tRNA species (B) and localization analysis of the C-terminus of A-PGS (C).
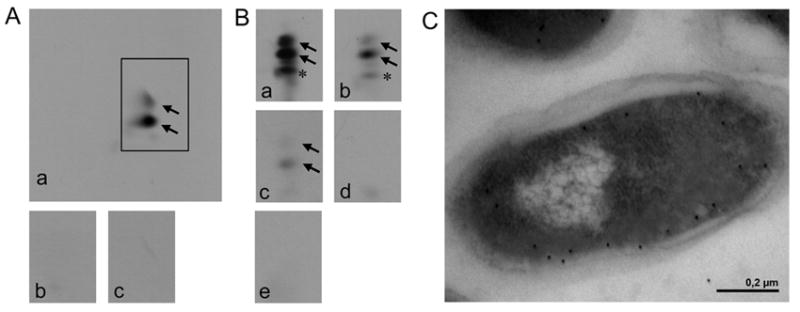
A. The activity of purified GST-A-PGS543-881 was analyzed by 2D-TLC of 14C-labelled phospholipids, A-PG formation is visualized by autoradiography. In vitro assay for GST-A-PGS543-881: a crude cellular extract from E. coli Rosetta (DE3) pLysS cells overproducing AlaRS enables the synthesis of Ala-tRNAAla (panel a); control reactions in the absence of A-PGS or after RNase A pre-treatment (panel b and c) are shown.
B. Modified in vitro assay for GST-A-PGS543-881 in the presence of recombinantly purified AlaRS enables the analysis of different tRNA substrates: a tRNA mixture from E. coli (panel a), purified tRNAAla1 or tRNAAla2 from P. aeruginosa which was synthesized by in vitro transcription (panel b or c). Control reactions in the absence of A-PGS or in the presence of RNase A (100 μg/ml) are shown (panel d or e). Formation of 2’ and 3’A-PG is highlighted by black arrows. * This additional lipid spot is observed due to the heterogeneity of the employed egg yolk lecithin lipid fraction. It is proposed that this is a result of the A-PGS dependent aminoacylation of a partly hydrolyzed PG molecule. Experiments using different sources of PG did not result in any compound migrating onto this specific position of the 2D-TLC.
C. The subcellular localization of a myc epitope C-terminally fused to the A-PGS was immune electron microscopically detected. Immunogold labelling was performed in the presence of a myc-specific antibody. Gold particles, indicative for the localization of the myc epitope, almost exclusively decorate the cytosolic part of the inner membrane.
