Abstract
Hydroxysteroid sulfotransferase SULT2A1 catalyzes the sulfation of hydroxysteroids and xenobiotics. It plays an important role in the detoxification of hydroxyl-containing xenobiotics and in the regulation of the biological activities of hydroxysteroids. ERRα is an orphan member of the nuclear receptor superfamily that is closely related to estrogen receptor alpha (ERα). Here we report that the mRNA expression of human SULT2A1 was suppressed by ERRα in Hep G2 cells. To investigate the mechanisms of this regulation, the effects of ERRα on human SULT2A1 promoter transcription in Hep G2 cells were investigated. Reporter luciferase assay results showed that ERRα significantly represses human SULT2A1 promoter transcription in Hep G2 cells. Deletion analysis indicated that human SULT2A1 promoter region between positions −188 and −130 is necessary for its repression by ERRα in Hep G2 cells. The 5’ DNA −188 to −130 region of human SULT2A1 contains IR2 and DR4 hormone response elements and two putative ERRα response elements (ERREs) (ERRE188: GCAAGCTCA and ERRE155: ATAAGTTCA). Interestingly, ERRE188 overlaps with the IR2 element and ERRE155 overlaps with the DR4 element. Our further investigation demonstrated that ERRα represses human SULT2A1 promoter transcription by competing with other nuclear receptors for binding to IR2 or DR4 elements. The interaction of ERRE188 and ERRE155 elements with ERRα was confirmed by electrophoretic mobility shift assay (EMSA) and chromatin immunoprecipitation (ChIP) analysis. Our results suggest that ERRα may play an important role in regulating the metabolism of drugs and xenobiotics and in regulating endogenous hydroxysteroid activities via the regulation of SULT2A1.
Keywords: Sulfotransferase, Estrogen-related receptor, SULT2A1, Gene regulation, Hep G2 cells
1. Introduction
Estrogen-related receptors (ERRs) are a subfamily of the orphan nuclear receptor superfamily [5, 46]. ERRs are composed of three members: ERRα, ERRβ, and ERRγ. ERRs share a high degree of sequence homology with estrogen receptors alpha (ERα) and beta (ERβ). ERRs and ERs are structurally related nuclear receptors, as they both bind to estrogen response elements, but are functionally different, as natural estrogens do not activate ERRs [21]. ERRα, designated NR3B1, is more closely related to ERα and ERβ than any other nuclear receptor members [19]. To date, no endogenous ligands of ERRα have been discovered, but ERRα shows constitutive activity without the presence of a ligand [10]. ERRα is widely expressed in adult tissues and is highly distributed in tissues that are involved in energy metabolism, including heart, skeletal muscle, kidney, intestine, and cerebellum [6]. In addition, ERRα also is transcribed in many kinds of tumors, such as gastrointestinal tumor, breast (mammary gland) tumor, glioma, endometrial tumor, lung tumor, and lymphoma [2, 13, 17, 37, 38, 42, 43].
Nuclear receptors function by binding to specific chromic DNA elements in their target genes. ERRα binds to the estrogen response element (ERE), which consists of an inverted repeat IR3 (AGGTCANNNTGACCT); directly competes with ERα for binding ERE; and modulates ERE-dependent transcription [41, 46, 47]. In addition, ERRα also binds to ERR response element (ERRE), which consists of an extended half site with the consensus sequence TNAAGGTCA [3, 34]. Many ERRE-containing genes that play a role in energy homeostasis and cancer have been shown to be the target of ERRα. These kind of ERRα-targeted genes include NO synthase [35], lactoferrin [46], osteopontin [40, 48], medium-chain acyl coenzyme A dehydrogenase (MCAD) [33], and thyroid hormone receptor genes [7]. ERRα is the repressor or activator of the promoter of these genes.
Sulfotransferases (SULTs) are one of the major families of phase II drug-metabolizing enzymes that catalyze the sulfation of hydroxyl-containing compounds. These include endogenous and exogenous molecules, ranging from peptides, neurotransmitters, and hormones to drugs and xenobiotics. Hydroxysteroid SULTs are a subfamily of SULTs. SULT2A1 is the major isoform of hydroxysteroid SULTs [39] and is highly expressed in liver and adrenal gland. SULT2A1 specifically sulfates hydroxysteroid dehydroepiandrosterone (DHEA) [28] and also catalyzes the sulfation (metabolism) of toxic bile acids such as lithocholic acid (LCA) [8, 25]. SULT2A1 has been reported to be a target of ERRα [30]. The expression of ERRα in the adrenal gland and its regulation of SULT2A1 suggest that ERRα plays an important role in the regulation of adrenal steroid production. It remains unknown whether ERRα affects SULT2A1 promoter activity in biotransformation organs and interferes with SULT2A1-mediated xenobiotics detoxification and regulation of hydroxysteroid activities. Hep G2 is a perpetual cell line derived from the liver tissue of a 15 year old Caucasian American male with a well differentiated hepatocellular carcinoma. Hep G2 cells are a suitable in vitro model system for the study of polarized human hepatocytes and for the study of drug metabolism. In the present study, ERRα-mediated human SULT2A1 promoter transcription in Hep G2 cells was investigated.
2. Materials and methods
2.1. Materials
Rifampicin, GW3945, and 1,25-hydroxy-vitamin D3 were from Fisher Scientific. DNA restriction enzyme was from Promega (Madison, WI). Lipofectamine™ 2000 was from Invitrogen (Carlsbad, CA). The plasmid extraction kit, DNA gel extraction kit, and total RNA extraction kit were from QIAGEN (Carlsbad, CA). Protein assay reagent was from Bio-Rad (Hercules, CA). The DIG gel shift kit, 2nd generation was from Roche Applied Science (Indianapolis, IN). Sonicated salmon sperm DNA was from Amersham Biosciences (Piscateway, NJ). Anti-human ERRα primary antibody was from Abcam (Cambridge, MA), and Protein A agarose was from Santa Cruz Biotechnology (Santa Cruz, CA).
2.2. Quantitative real-time PCR determining SULT2A1 mRNA expression
Hep G2 cells were seeded onto six wells plate and reached about 80% confluence before transfection experiments. CAR, ERRα, or empty vector was transfected into cells by Lipofectamine™ 2000. After 48 hours, the cells were collected, and then total RNA were isolated using TRI reagent per manufacturer instructions. cDNA were synthesized from total RNA using random primers. Quantitative real-time PCR was employed to determine the effect of CAR and ERRα on SULT2A1 mRNA expression in Hep G2 cells. PCR was performed using QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA) following the manufacturer's instruction. Primers were designed with Primer Express as follow: hACTBF321: 5’-AGAAAATCTGGCACCACACC-3’, hACTBR462: 5’-GGGGTGTTGAAGGTCTCAAA-3’; hSULT2A1F163: 5’-TGAGTTCGTGATAAGGGATGAA-3’, hSULT2A1R294: 5’-CAGATGGGCACAGATTGGAT-3’. Real-time PCR was performed on ABI PRISM 7500 (Applied Biosystems, Foster City, CA). Initially, regular PCR products were purified with GENECLEAN Turbo (Qbiogene, Carlsbad, CA) for constructing standard curves (10–108 copies). A standard curve was plotted with the threshold cycle (CT) versus the logarithmic value of the gene copy number. The gene copy number of unknown samples was generated directly from the standard curve by the software Sequence Detector 1.7. At least two repeats were run for each sample; each experiment was repeated twice. SULT2A1 mRNA copy number was normalized to human β-actin mRNA.
2.3. Preparation of hSULT2A1 promoter reporter plasmids
The 5’-flanking region DNA (−1463 to +48) of hSULT2A1 gene was inserted upstream of the firefly luciferase vector pGL3-Basic, as previously described [11]. The SULT2A1 deletion constructs used in the present study (−713/+48, −444/+48, −235/+48, −188/+48, −130/+48, and −65/+48) were described previously [11]. Three more deletion constructs (−163/+48, −44/+48, and −37/+48) were generated by PCR for this study using the −1463 to +48 fragment of the hSULT2A1 gene as a template. The products were inserted into the Mlu I and Xhol I sites of the pGL3-Basic vector. The following 5’ primers were used: for construct −163/+48, 5’-CCTGAACGCGTCTCTAGATAAGTTC-3’; for construct −44/+48, 5’-GTGACGCGTAAAGATCGTTTTATCCTTG-3’; for construct −37/+48, 5’-GGTTACAACGCGTTTTATCCTTGCTG-3’. The 3′ primers were identical for all the constructs (5′-ATTCTCGAGGCGTGGTGTGAGGGTTTC-3′).
Construct 3XIR2-TK-Luc, which contained three copies of IR2 element, was generated by cloning three tandem repeats of IR2 (-ACGCAAGCTCAGATGACCCCT-) into the 5’ end of the TK promoter of the TK-Luc vector. Construct 3XDR4-TK-Luc, which contained three copies of DR4 element, was generated by cloning three tandem repeats of DR4 (-AGATAAGTTCATGATTGCTCAACA-) into the 5’ end of the TK promoter. DNA sequencing at the Oklahoma State University core facility verified all of the above constructs. Tk-Luc vector was a generous gift from Professor Bandana Chatterjee of the University of Texas Health Science Center at San Antonio.
2.4. Transfections and reporter gene assays in Hep G2 cells
Hep G2 cells, a human hepatocellular liver carcinoma cell line, were purchased from ATCC (Manassas, VA). Hep G2 cells were grown and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma)/nutrient mixture F-12 ham (Sigma) supplemented with 10% fetal bovine serum (FBS). For transfection, cells were seeded onto six-well plates at a density of 3×105 cells per well. After 16 h, they were transfected with 1000 ng of reporter plasmid, 200 ng of nuclear receptor expression vector, and 120 ng of the pRL-TK plasmid (Promega). The transfection agents contained 200 µl of Opti-MEM and 4 µl of Lipofectamine™ 2000 (Invitrogen). The pRL-TK plasmid, which expresses Renilla luciferase, was used as an internal standard for normalizing luciferase activity. PUC vector DNA was used as an empty vector to keep the total transfected DNA at a fixed value. Twelve hours after transfection, nuclear receptor agonists for hCAR, hPXR, hVDR, or hLXRα were added at a final concentration of 0.1 µM CITCO, 10 µM rifampicin, 10 nM 1,25-hydroxy-vitamin D3, and 2 µM GW3965, respectively. Forty-eight hours after transfection, cells were collected and firefly and Renilla luciferase activities were measured using the Dual-Luciferase® Reporter Assay System (Promega). Each experiment was repeated three times, with each performed in duplicate. Results are given as means ± SD.
2.5. Electrophoretic mobility shift assay (EMSA) and super shift assay
Nuclear extracts were prepared from Hep G2 cells transfected for 48 h with either hCAR or hERRα receptor. Cells were harvested by releasing the cells from monolayer cultures via scraping. Suspended cells were then lysed by subjecting them to three freeze-thaw cycles in buffer containing 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DDT, and proteinase inhibitor. The crude nuclear pellet was obtained by centrifugation for a few seconds. The pellet was then extracted in nuclear extraction buffer containing 20 mM HEPES (pH 7.9), 25% glycerol, 1.5 mM MgCl2, 420 mM KCl, 0.5 mM DDT, 0.2 mM EDTA, and proteinase inhibitor. After 2 h of incubation on ice, the mixture was centrifuged, leaving the resulting nuclear extracts in the supernatant. Protein concentrations were measured with a Bradford assay [27, 32].
EMSA was performed using a DIG gel shift kit according to the manufacturer’s instructions. Oligonucleotide DNA probes containing a nuclear receptor response element were labeled with digoxigenin-11-ddUTP. The probes included IR2 and ERRE188 (5′-CTCAGGAACGCAAGCTCAGATGACCCCTAAAATGGT -3′); DR4 and ERRE155 (5’-CTCTAGATAAGTTCATGATTGCTCAACATCTTCAATC-3’). The standard gel shift binding reaction (20 µl) contained 20 mM Hepes (pH 7.6), 1 mM EDTA, 10 mM (NH4)2S04, 1 mM DTT, 0.2 % (w/v) Tween 20, 30 mM KCl, 1 µg poly[d(I-C)], 1 µg poly L-lysine, and 2 µg Hep G2 nuclear extract. After the addition of double-stranded oligonucleotide probe (0.4 ng), reactions were incubated at room temperature for 20 min. Competitions were performed with 125-fold molar excess of unlabeled oligonucleotides.
In the super shift assay, the nuclear extract was pre-incubated with antibody at room temperature for 20 min before the addition of DNA probes. Antibody against hCAR (sc-8541) was from Santa Cruz Biotechnology (Santa Cruz, CA), and antibody against hERRα was from Abcam (Cambridge, MA). Protein-DNA complexes were resolved on pre-electrophoresed 5% native polyacrylamide gels in 0.5× Tris/borate/EDTA (TBE) buffer at room temperature and then blotted onto positively charged nylon membranes. The digoxigenin-labeled oligonucleotides were visualized through an enzymatic immunoassay using anti-digoxigenin-AP Fab-fragments and the chemiluminescence substrate CSPD. The generated hemiluminescence signals were recorded with a VersaDoc imaging system (Bio-Rad, Hercules, CA).
2.6. Chromatin immunoprecipitation (ChIP)
Hep G2 cells were seeded onto 10-cm plates at a density of 5 × 106 cells per plate, and were transfected with either 6 µg of pCDNA-hERRα or pCDNA control plasmid. Forty-eight hours after transfection, the cells were cross-linked with 1% formaldehyde at room temperature for 10 min. Cross-linking was stopped by adding glycine to a final concentration of 125 mM. Cells harvested by scraping were then lysed, and chromatin was sonicated to an average size of 500 bp. Diluted chromatin suspensions were pre-cleared with sonicated salmon sperm DNA and protein A agarose slurry for 2 h at 4°C. Supernatants were collected and processed in one of two ways: (1) They were incubated at 4°C overnight with either 4 µg of anti-human ERRα antibody (Abcam) or normal rabbit IgG (Santa Cruz Biotechnology); or (2) they were directly processed in the next step, which lacked antibody (negative control). Normal rabbit IgG was used as a negative control. After immunoprecipitation, salmon sperm DNA/protein A agarose slurry was added, and the mixture was incubated for another 1 h. Precipitates were collected, washed, eluted, and then cross-linkages were reversed at 65°C overnight. The uncross-linked chromatins were digested with proteinase K at 45°C for 1 h. Chromatin DNAs were purified by phenol chloroform extraction and precipitated by ethanol. DNAs were amplified by PCR using GoTaq polymerase (Promega).
Two potential ERRα-binding regions (−235/−119) of SULT2A1 promoter and its upstream region (−5000/−4750) lacking the ERRα response element were selected for amplification. The amplification regions and primers were as follows:
−235/−119 (sense: 5’-TTGTCCTCGTGTTTGTTATTCG-3’; antisense: 5’-GACCCATACTCAAAAGATTGAAG-3’); −5000/−4750 (sense: 5’-GGCTATCATTTTGCCCTATGGC-3’, antisense: 5’-ACAGAATGTTAGAGGAACAGGC-3’).
2.7. Statistical analysis
One-way ANOVA followed by the Dunnett’s test was used to calculate the statistical significance of the difference between the control group means and treated group means. In all cases, P<0.05 was considered significant and P<0.01 was considered very significant. Data presented in the figures are means ± standard deviation (SD). All the transfection and activity assays were performed in duplicate and were repeated at least two times.
3. Results
3.1 Effect of ERRα on SULT2A1mRNA expression in Hep G2 cells
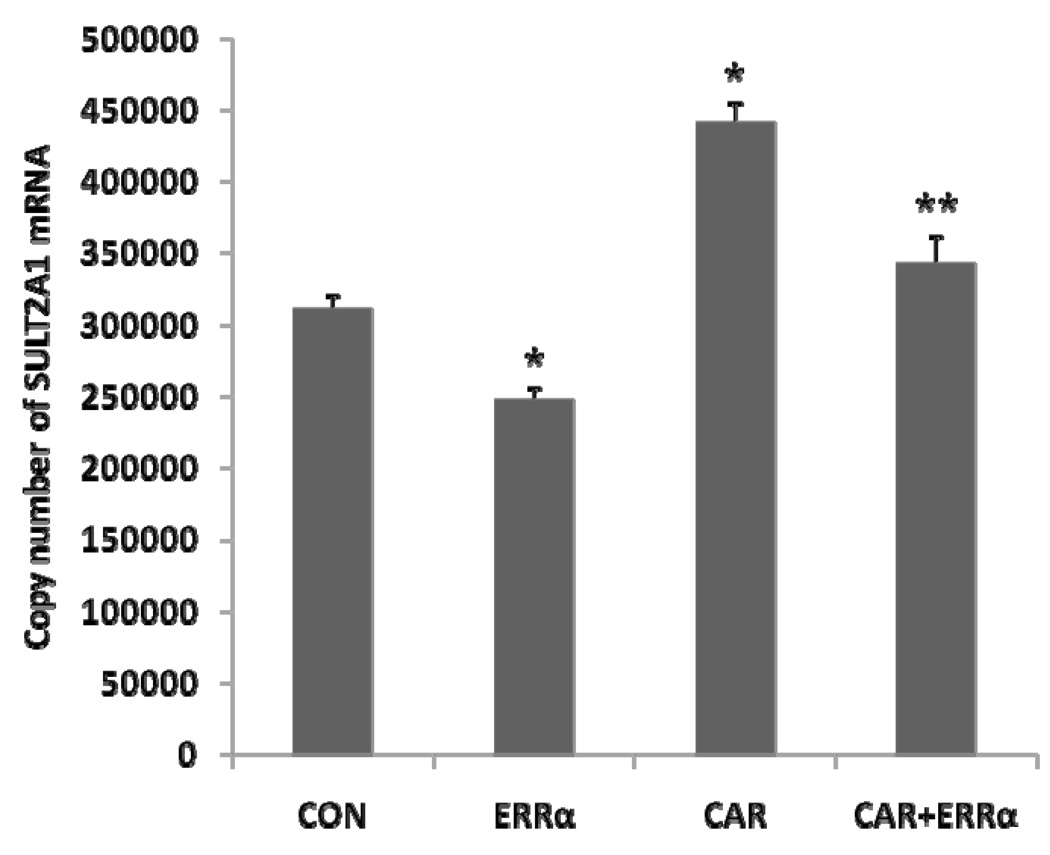
The results in Figure 1 suggested that ERRα over expression suppressed human SULT2A1 mRNA expression in Hep G2 cells (Figure 1). Constitutive androstane receptor (CAR) is known to mediate the induction of SULT2A1 expression [1, 9, 12, 14, 22, 29, 31, 45]. When co-transfected with CAR, ERRα also suppressed CAR activation of SULT2A1 expression in Hep G2 cells (Figure 1). The SULT2A1 mRNA expression was determined by real time PCR. The direct effect of ERRα on SULT2A1 expression in Hep G2 cells has not been reported.
Figure 1. Effect of ERRα on SULT2A1mRNA expression.
Hep G2 cells were co-transfected with 500 ng ERRα vector and either 500 ng CAR or control vector (Final concentration: 1µg per well) in six-well plate. SULT2A1 mRNA expression was determined by absolute quantitative Real time PCR, SULT2A1 mRNA copy number was normalized to human β-actin mRNA. *p<0.05, compared with control; **p<0.01 compared with CAR.
3.2. ERRα effect on SULT2A1 promoter activity in Hep G2 cells
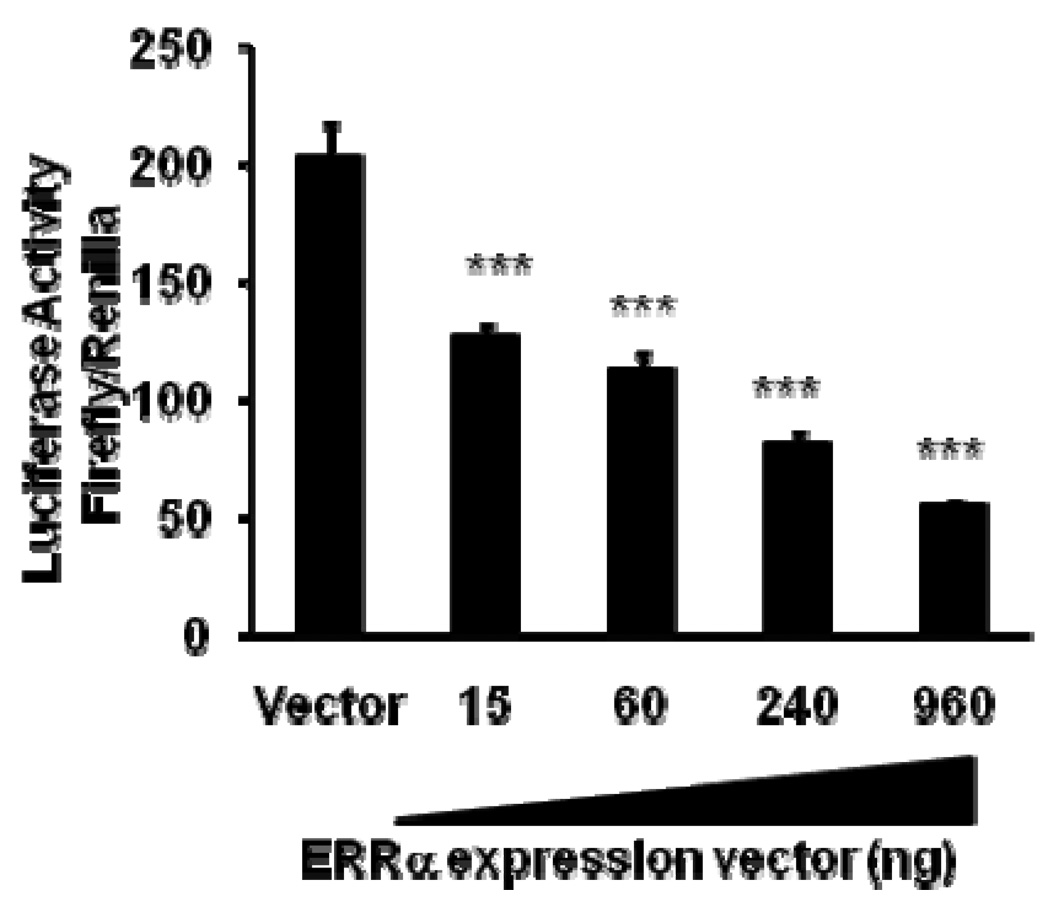
To investigate the mechanisms of the effect of ERRα on SULT2A1 expression in Hep G2, we constructed a SULT2A1 promoter luciferase reporter by inserting the 5’-flanking region DNA (−1463 to +48) of the hSULT2A1 gene into the region upstream of the firefly luciferase vector pGL3-Basic. As shown in Figure 2, ERRα overexpression inhibited SULT2A1 promoter activity in Hep G2 cells in a concentration-dependent manner.
Figure 2. Effect of ERRα overexpression on human SULT2A1 promoter transcription in Hep G2 cells.
Hep G2 cells were co-transfected with human SULT2A1 (−1463/+48) luciferase promoter construct (1 µg/well) and a pCDNA3.0-ERRα expression plasmid. The control was co-transfected with the SULT2A1 luciferase promoter construct and an expression vector lacking ERRα. Data were normalized according to Renilla luciferase activity. Results are presented as means ± SD from three independent transfection experiments performed in duplicate. ***p<0.001, compared with basal SULT2A1 reporter activity of cells transfected with empty vector.
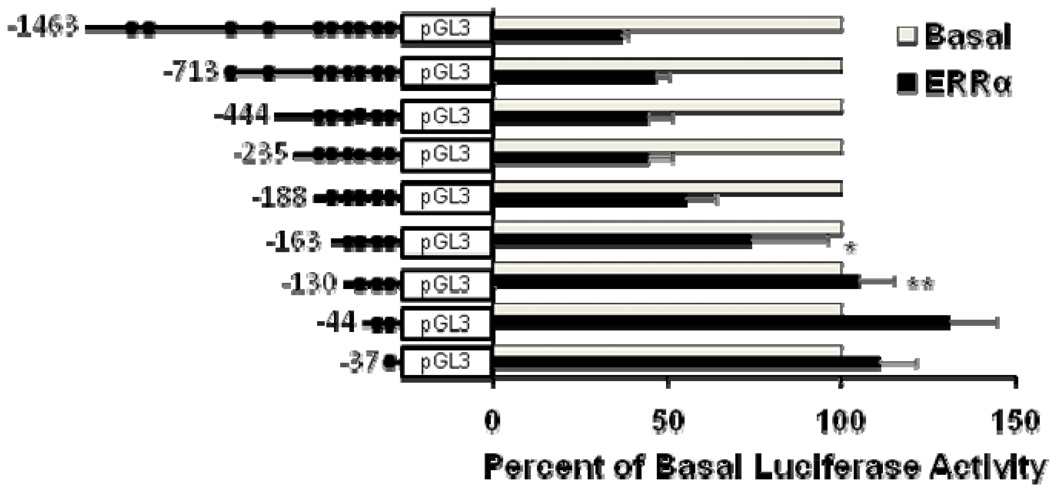
To determine which specific region of the hSULT2A1 promoter is responsible for ERRα-mediated repression in Hep G2 cells, we created a series of truncated SULT2A1 promoter constructs. Each truncated construct in combination with either pCDNA-ERRα expression vector or pCDNA empty vector were co-transfected into Hep G2 cells. As shown in Figure 3, ERRα-mediated repression of −713/+48, −444/+48, −235+48, −188/+48 SULT2A1 promoter activity did not significantly change, but the repression of −163/+48 and −130/+48 SULT2A1 promoter activity were significantly reduced compared with −1463/+48 SULT2A1 promoter activity. The deletion analysis indicated that the SULT2A1 promoter region located between positions −188 and −130 is necessary for its repression by ERRα in Hep G2 cells.
Figure 3. Deletion analysis identifying ERRα-binding region in human SULT2A1 promoter.
Hep G2 cells were co-transfected with pCDNA3.0- ERRα expression plasmid and luciferase constructs containing truncated segments of SULT2A1 promoter having different lengths. Data were normalized according to Renilla luciferase activity. The percentage of inhibition was calculated relative to the basal promoter control. Results are presented as means ± SD from three independent transfection experiments performed in duplicate. *p<0.05, **p<0.01, compared with −1463/+48 SULT2A1 promoter construct.
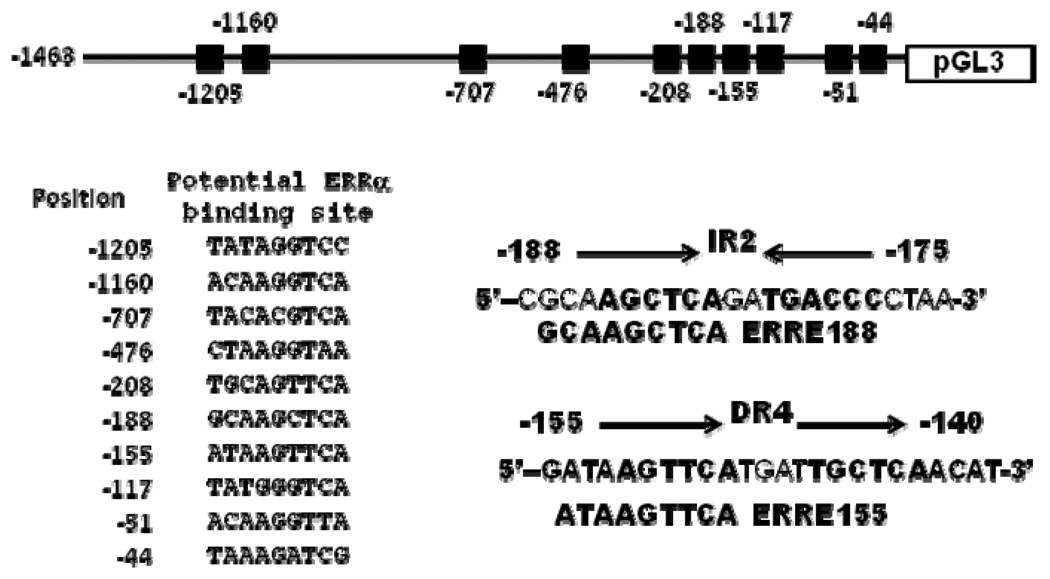
The computer sequence analysis results shown in Figure 4 revealed that the SULT2A1 5’-flanking DNA region located between positions −188 and −130 contained IR2 and DR4 nuclear receptor response elements, and two putative ERREs (ERRE188: GCAAGCTCA and ERRE155: ATAAGTTCA) that closely matched the consensus ERRE half site TCAAGGTCA. In addition, ERRE188 overlapped with IR2 element, and ERRE155 overlapped with DR4 element.
Figure 4. Schematic representation of SULT2A1 promoter showing putative ERRα-binding sites and IR2 and DR4 elements.
3.3. ERRα modulation of SULT2A1 promoter transcription mediated by ERRE188 and ERRE155 elements in Hep G2 cells
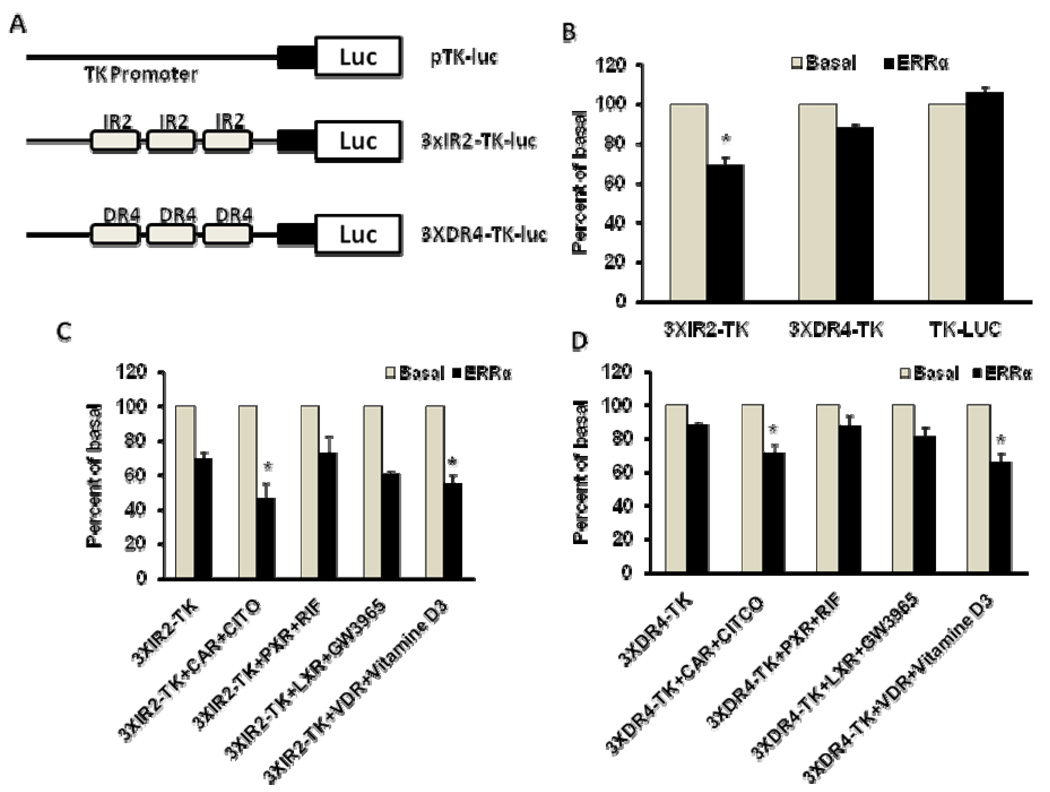
To investigate whether ERRα directly interacts with the ERRE188 element (which overlaps with IR2) and ERRE155 element (which overlaps with DR4), we constructed 3XIR2-TK-Luc, a reporter plasmid containing three copies of the IR2 element cloned upstream of the TK promoter (Figure 5A), and 3XDR4-TK-Luc, a reporter plasmid containing three copies of DR4 element also cloned upstream of the TK promoter (Figure 5A). ERRα efficiently repressed the activity of 3XIR2-TK-Luc but weakly inhibited the activity of 3XDR4-TK-Luc (Figure 5B).
Figure 5. ERRα modulation of SULT2A1 promoter transcription mediated by IR2 and DR4 elements in Hep G2 cells.
A. Schematic representations of pTK-luc, 3XIR2-TK-luc, and 3XDR4-TK-luc reporter plasmids. B. ERRα modulation of IR2 and DR4 reporter transcription in Hep G2 cells. Cells were co-transfected with pTK-luc, 3XIR2-TK-luc, or 3XDR4-TK-luc reporter plasmids (1 µg/well) and with either pCDNA3.0-ERRα expression plasmid (0.25 µg/well) or pCDNA3.0(0.25 µg/well). Data were normalized according to Renilla activity. C. ERRα modulation of nuclear receptor-mediated IR2 reporter transcription in Hep G2 cells. Hep G2 cells were co-transfected with 3XIR2-TK-luc reporter plasmids (1 µg/well) and pCDNA3.0-ERRα expression plasmid (0.25 µg) and nuclear receptor (0.25 µg; CAR, PXR, VDR or LXRα). D. ERRα modulation of nuclear receptor-mediated DR4 reporter transcription in Hep G2 cells. Hep G2 cells were co-transfected with 3XDR4-TK-luc reporter plasmids (1 µg/well) and pCDNA3.0-ERRα expression plasmid (0.25 µg) and nuclear receptor (0.25 µg; CAR, PXR, VDR, or LXRα). Data were normalized according to Renilla activity. The percentage of inhibition was calculated relative to the basal promoter control. Results are presented as means ± SD from three independent transfection experiments performed in duplicate. *p<0.05, compared with the basal activity of 3XIR2-TK promoter (4B), 3XIR2-TK (4C), or 3XDR4-TK (4D).
Nuclear receptors PXR, CAR, VDR, and LXR have been shown to regulate SULT2A1 promoter transcription mediated by either IR2 or DR4 elements [11, 15, 23, 31]. To determine whether ERRα affects nuclear-receptor-modulated SULT2A1 promoter transcription mediated by IR2 or DR4 elements, we co-transfected Hep G2 cells with either plasmid 3XIR2-TK-Luc or 3XDR4-TK-Luc and ERRα and a nuclear receptor (CAR, PXR, VDR or LXR). Twelve hours after transfection, respective nuclear receptor agonists were added to the cultures: 0.1 µM CITCO for CAR, 10 µM rifampicin for PXR, 10 nM 1,25-hydroxy-vitamin D3 for VDR, and 2 mM GW3965 for LXR. ERRα significantly repressed CAR- and VDR-mediated 3XIR2-TK-Luc transcription. Similarly, ERRα also significantly repressed CAR- and VDR-mediated 3XDR4-TK-Luc transcription.
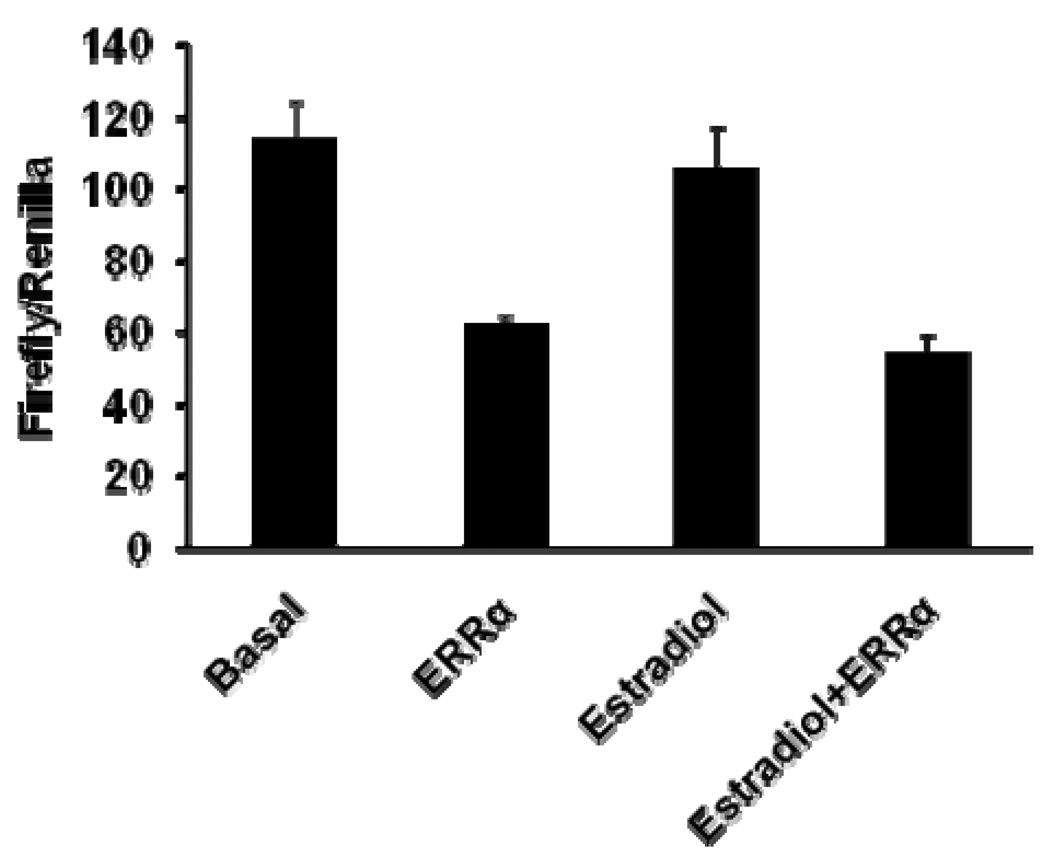
ERRα down-modulates estrogen-receptor-stimulated transcription [36, 48]. To investigate whether the cell-type-dependent ERRα-modulated SULT2A1 promoter transcription we observed in Hep G2 cells is related to estrogen, we added estradiol (final concentration of 10−8 M) to cell cultures 12 hours after transfection. Estradiol did not affect SULT2A1 promoter transcription in Hep G2 cells (Figure 6). In addition, estradiol did not affect ERRα-mediated repression of SULT2A1 promoter transcription.
Figure 6. Effect of estradiol and ERRα on SULT2A1 promoter transcription in Hep G2 cells.
SULT2A1 promoter (−1453/+48) reporter plasmids (1 µg/well) were co-transfected with pCDNA3.0-ERRα expression plasmid (0.25 µg/well) or with pCDNA3.0 (0.25 µg/well). After 12 hours, the cells were incubated with 10−8 M of estradiol or DMSO. Cells were collected 48 h after transfection, and firefly and Renilla luciferase activities were measured.
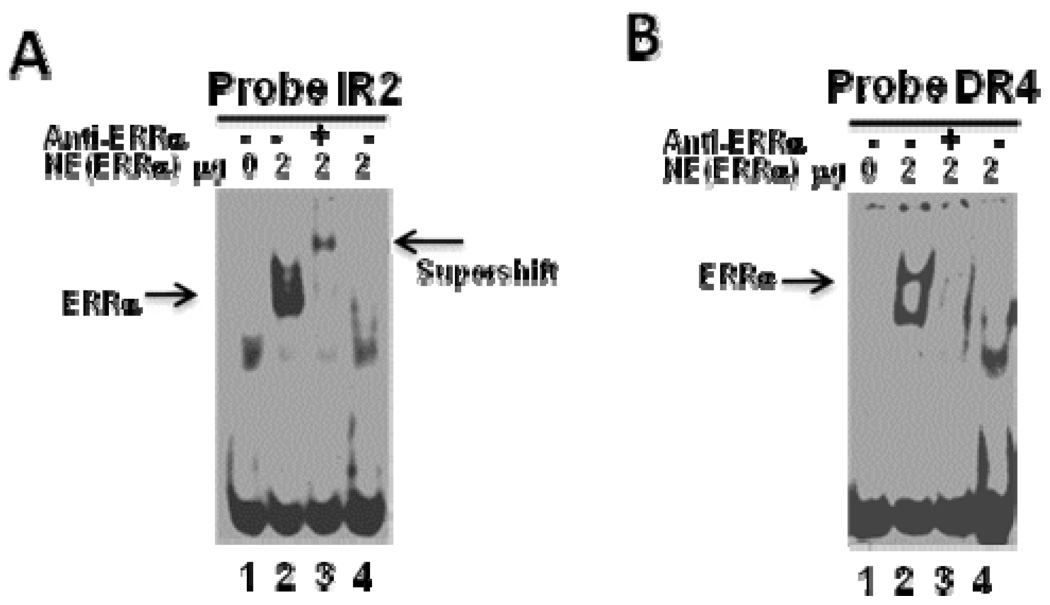
Computational prediction analyses indicated that SULT2A1 promoter contains several putative ERREs (Figure 4). Our deletion analysis revealed that ERRE188 and ERRE155 are located within the region responsible for ERRα-mediated repression of SULT2A1 transcription in Hep G2 cells. To confirm that ERRα binds regulatory elements in the SULT2A1 promoter, we performed EMSA using digoxigenin-labeled IR2 and DR4, and crude nuclear extracts obtained from Hep G2 cells overexpressing ERRα. EMSA demonstrated that ERRα binds both IR2 (overlap with ERRE188) and DR4 (overlap with ERRE155) (Figure 7). To check the specificity of this binding, we added a 125-fold molar excess of unlabeled competitor primers to the reaction mixture (lane 4). Unlabeled primers blocked the binding of the labeled primers, suggesting the specificity of this binding. We also performed supershift analysis with ERRα-specific antibodies. As shown in Figure 7A, IR2-ERRα protein complexes were retarded, forming a supershift band. Although DR4-ERRα protein complexes also were retarded and supershifted (Figure 7B), the retarded band was not detectable.
Figure 7. Electrophoretic mobility shift assay of ERRα binding to the SULT2A1 gene promoter.
A. ERRE188 (overlaps with IR2). B. ERRE155 (overlaps with DR4). Digoxigenin-labeled DNA sequence was incubated with 2 µg of nuclear extract obtained from Hep G2 cells transfected with either pCDNA (Lane 1) or pCDNA-ERRα (Lanes 2, 3, and 4). For the super shift assay, the nuclear extract was pre-incubated with anti-ERRα antibody at room temperature for 20 min before the addition of DNA probes (Lane 3). Competition was performed using 125-fold molar excess of unlabeled ERRE (Lane 4).
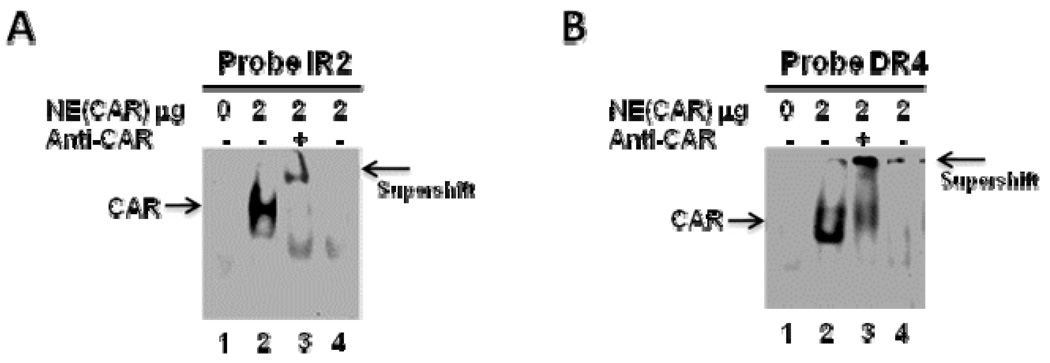
CAR has been shown to directly bind IR2 and DR4 [12, 14]. Our sequence analysis indicated that ERRE188 overlaps with IR2 element and that ERRE155 overlaps with DR4 element. Thus, we performed EMSA to determine whether CAR binds to the SULT2A1 IR2 and DR4 elements. As shown in Figure 8, CAR direct bound both IR2 and DR4.
Figure 8. Electrophoretic mobility shift assay of CAR binding to the SULT2A1 gene promoter.
A. ERRE188 (overlaps with IR2). B. ERRE155 (overlaps with DR4). Digoxigenin-labeled DNA sequence was incubated with 2 µg of nuclear extract obtained from Hep G2 cells transfected with either pCDNA (Lane 1) or pCDNA-CAR (Lanes 2, 3, and 4). For the super shift assay, the nuclear extract was pre-incubated with anti-CAR antibody at room temperature for 20 min before the addition of DNA probes (Lane 3). Competition was performed using 125-fold molar excess of unlabeled ERRE (Lane 4).
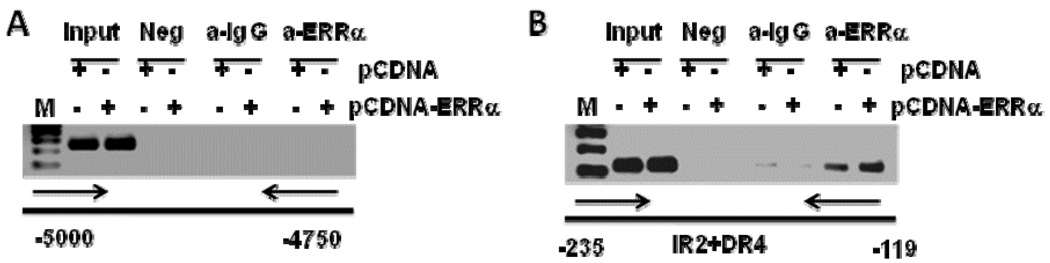
We performed a ChIP assay with the primer set used to amplify the −239/−119 region of SULT2A1 promoter to confirm that ERRα binds the −239/−119 region. This region contains putative ERRα-binding sites, ERRE188 and ERRE155. ChIP performed with a specific ERRα antibody confirmed that, in Hep G2 cells, ERRα and endogenous SULT2A1 promoter interact physiologically (Figure 9B). No reaction was observed with the control IgG (Figure 9B). Moreover, performing ChIP using a negative control primer set located 5000bp and 4750bp upstream of the SULT2A1 promoter did not result in a PCR product (Figure 9A).
Figure 9. ChIP assay to detect ERRα binding to the endogenous hSULT2A1 promoter region containing putative ERREs.
A. The upstream region (−5000/−4750) of the SULT2A1 promoter lacking ERRα response elements was selected for amplification. B. Potential ERRα-binding region (−235/−119) of the SULT2A1 promoter that contains putative ERREs (ERRE188 and ERRE155).
4. Discussion
We investigated the effect of ERRα on SULT2A1 gene promoter transcription in Hep G2 cells. Our data demonstrated that ERRα directly binds the SULT2A1 promoter region located between positions −188 and −130, and down-modulates SULT2A1 promoter transcription in Hep G2 cells. ERRα has been reported to upregulate SULT2A1 promoter transcription in CV-1 cells [30]. Taken together, these findings indicate that the effect of ERRα on SULT2A1 promoter transcription is cell-type dependent.
Sequence analyses showed that the SULT2A1 5’-flanking DNA region located between positions −188 and −130 contained IR2 and DR4 nuclear receptor response elements (Figure 4), and two putative ERREs (ERRE188: GCAAGCTCA and ERRE155: ATAAGTTCA) that closely match the consensus ERRE half site TCAAGGTCA (Figure 4). In addition, ERRE188 overlapped with IR2 element, and ERRE155 overlapped with DR4 element. Nuclear receptors CAR, PXR, VDR, LXR, and FXR have been reported to bind to either IR2 or DR4 elements and to modulate SULT2A1 promoter transcription [11, 15, 23, 31]. In the present study, EMSA demonstrated that ERRα interacts with both IR2 and DR4 elements (Figure 7A, 6B). Further investigation via ChIP assay revealed that ERRα directly binds the endogenous SULT2A1 promoter region that contains IR2 and DR4 elements (Figure 9). Our results suggest that ERRα represses SULT2A1 promoter transcription by competing with other nuclear receptors for binding to IR2 or DR4 elements. To confirm this hypothesized mechanism, we constructed reporter plasmid 3XIR2-TK-Luc, which contained three copies of IR2 cloned upstream of the TK promoter, and plasmid 3XDR4-TK-Luc, which contained three copies of DR4 cloned upstream of the TK promoter (Figure 5A). ERRα efficiently repressed the reporter activity of both 3XIR2-TK-Luc and 3XDR4-TK-Luc (Figure 5B). Furthermore, ERRα repressed VDR- and CAR-mediated 3XIR2-TK-Luc (Figure 5C) or 3XDR4-TK-Luc transcription (Figure 5D).
Previously, ERRα has been shown to modulate estrogen-receptor-mediated transcription in a cell-type-dependent manner [4, 26]. However, the present investigation in Hep G2 cells showed that estrogen failed to affect SULT2A1 promoter transcription (Figure 6). Moreover, estrogen failed to affect ERRα-mediated repression of SULT2A1 promoter transcription. Thus, other nuclear receptors or factors must contribute to the cell-type-dependent ERRα-mediated modulation of SULT2A1 promoter transcription. Consistent with this notion is our finding that ERRα significantly represses CAR- and VDR-mediated reporter 3XIR2-TK-Luc and 3XDR4-TK-Luc transcription in Hep G2 cells (Figure 5C, 5D). This indicates that VDR and CAR were at least partly responsible for the ERRα-modulated inhibition of SULT2A1 promoter transcription in Hep G2 cells.
SULTs are one of the major families of phase II drug-metabolizing enzymes that catalyze the sulfation of hydroxyl-containing compounds. SULT2A1 is the major isoform of hydroxysteroid SULTs. SULT2A1 is responsible for the in vivo sulfation of DHEA; therefore, ERRα in the adrenal gland has been suggested to play an important role in the regulation of adrenal steroid production [30]. In the present study, we observed that ERRα represses SULT2A1 promoter transcription in Hep G2 cells. Besides DHEA sulfation, SULT2A1 is also involved in the clearance of toxic bile acids such as lithocholic acid (LCA) and xenobiotic toxicants in the liver. Thus, ERRα may have an important function in regulating drug and xenobiotic metabolism. Elucidating the gene regulation mechanisms of SULTs will contribute to our understanding of the biological functions of SULTs and will facilitate the development of effective drugs for certain diseases.
ERRα is known to be involved in the regulation of genes that play roles in the energy metabolism network [18]. Gene expression profile in Hep G2 cells induced by PGC-1α through ERRα have demonstrated that ERR is a key regulator of oxidative phosphorylation and other related energy metabolism pathways in this cell [16]. SULT2A1, which catalyzes the metabolism of steroid hormone and bile acids, is highly expressed in liver. Bile acids facilitate the absorption of lipids and fat-soluble vitamins, and the elimination of cholesterol [20], and they also induce energy expenditure by promoting intracellular thyroid hormone activation[44]. SULT2A1 control the bile acid pool by sulfating hydrophobic secondary bile acids [24]. SULT2A1 may have an integrated role in energy metabolism cascade in hepatic cells. We found that SULT2A1 is the target of ERRα in Hep G2 cells. Our results suggest that ERRα may perform its role in regulating energy metabolism via SULT2A1. SULT2A1 is also the major SULT to detoxify alcoholic xenobiotics. ERRα may affect drug and xenobiotic metabolism in hepatic cells.
Literature reports and our results suggested that SULT2A1 can be regulated through different nuclear receptors and the regulation mechanisms can be different for different nuclear receptors [11, 15, 23, 31]. The regulation of SULT2A1 by the same nuclear receptor is also cell-type dependent. The in vivo regulation of SULT2A1 may be complex. The final changes of SULT2A1 activity will be the dependent on the relative expression levels of different nuclear receptors and the levels of agonists of nuclear receptors existing in vivo in the specific cell type. Our results have suggested that SULT2A1 regulation mediated through ERRα is cell-type dependent [30]. Based on literature reports and our results, SULTs are usually not as extensively regulated by xenobiotic drugs compared to other drug metabolizing enzymes such as drug metabolizing P-450s, the multiple gene regulatory mechanisms of SULT2A1 may be part of the reseaon.
Acknowledgements
We are grateful to Dr. Jianrong Lu, Shands Cancer Center, University of Florida, for the pCDNA-ERRα plasmid gift. This work was supported in part by National Institutes of Health (NIH) grant GM078606 (G.C.), American Cancer Society (ACS) grant RSG-07-028-01-CNE (G.C.), United States Department of Agriculture (USDA) grant 2006-35200-17137 (G.C.), and Oklahoma Center for the Advancement of Science and Technology (OCAST) grant HR05-015 (G.C.).
Abbreviations
- SULT
sulfotransferase
- ERR
estrogen-related receptor
- DHEA
dehydroepiandrosterone
- SULT2A1
hydroxysteroid sulfotransferase (DHEA-ST)
References
- 1.Alnouti Y, Klaassen CD. Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther. 2008;324:612–621. doi: 10.1124/jpet.107.129650. [DOI] [PubMed] [Google Scholar]
- 2.Ariazi EA, Clark GM, Mertz JE. Estrogen-related receptor alpha and estrogen-related receptor gamma associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer research. 2002;62:6510–6518. [PubMed] [Google Scholar]
- 3.Barry JB, Laganiere J, Giguere V. A single nucleotide in an estrogen-related receptor alpha site can dictate mode of binding and peroxisome proliferator-activated receptor gamma coactivator 1alpha activation of target promoters. Molecular endocrinology (Baltimore, Md. 2006;20:302–310. doi: 10.1210/me.2005-0313. [DOI] [PubMed] [Google Scholar]
- 4.Bonnelye E, Merdad L, Kung V, Aubin JE. The orphan nuclear estrogen receptor-related receptor alpha (ERRalpha) is expressed throughout osteoblast differentiation and regulates bone formation in vitro. The Journal of cell biology. 2001;153:971–984. doi: 10.1083/jcb.153.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnelye E, Vanacker JM, Spruyt N, Alric S, Fournier B, Desbiens X, Laudet V. Expression of the estrogen-related receptor 1 (ERR-1) orphan receptor during mouse development. Mechanisms of development. 1997;65:71–85. doi: 10.1016/s0925-4773(97)00059-2. [DOI] [PubMed] [Google Scholar]
- 6.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castet A, Herledan A, Bonnet S, Jalaguier S, Vanacker JM, Cavailles V. Receptor-interacting protein 140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Molecular endocrinology (Baltimore, Md. 2006;20:1035–1047. doi: 10.1210/me.2005-0227. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee B, Echchgadda I, Song CS. Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. Methods in enzymology. 2005;400:165–191. doi: 10.1016/S0076-6879(05)00010-8. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee B, Echchgadda I, Song CS. Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. Methods Enzymol. 2005;400:165–191. doi: 10.1016/S0076-6879(05)00010-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Zhou D, Yang C, Sherman M. Molecular basis for the constitutive activity of estrogen-related receptor alpha-1. The Journal of biological chemistry. 2001;276:28465–28470. doi: 10.1074/jbc.M102638200. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Maiti S, Zhang J, Chen G. Nuclear receptor interactions in methotrexate induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1) Journal of biochemical and molecular toxicology. 2006;20:309–317. doi: 10.1002/jbt.20149. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Zhang J, Baker SM, Chen G. Human constitutive androstane receptor mediated methotrexate induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1) Toxicology. 2007;231:224–233. doi: 10.1016/j.tox.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung CP, Yu S, Wong KB, Chan LW, Lai FM, Wang X, Suetsugi M, Chen S, Chan FL. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. The Journal of clinical endocrinology and metabolism. 2005;90:1830–1844. doi: 10.1210/jc.2004-1421. [DOI] [PubMed] [Google Scholar]
- 14.Echchgadda I, Song CS, Oh T, Ahmed M, De La Cruz IJ, Chatterjee B. The Xenobiotic-Sensing Nuclear Receptors Pregnane X Receptor, Constitutive Androstane Receptor, and Orphan Nuclear Receptor Hepatocyte Nuclear Factor 4{alpha} in the Regulation of Human Steroid-/Bile Acid-Sulfotransferase. Mol Endocrinol. 2007;21:2099–2111. doi: 10.1210/me.2007-0002. [DOI] [PubMed] [Google Scholar]
- 15.Fang HL, Strom SC, Ellis E, Duanmu Z, Fu J, Duniec-Dmuchowski Z, Falany CN, Falany JL, Kocarek TA, Runge-Morris M. Positive and negative regulation of human hepatic hydroxysteroid sulfotransferase (SULT2A1) gene transcription by rifampicin: roles of hepatocyte nuclear factor 4alpha and pregnane X receptor. The Journal of pharmacology and experimental therapeutics. 2007;323:586–598. doi: 10.1124/jpet.107.124610. [DOI] [PubMed] [Google Scholar]
- 16.Gaillard S, Grasfeder LL, Haeffele CL, Lobenhofer EK, Chu TM, Wolfinger R, Kazmin D, Koves TR, Muoio DM, Chang CY, McDonnell DP. Receptor-selective coactivators as tools to define the biology of specific receptor-coactivator pairs. Mol Cell. 2006;24:797–803. doi: 10.1016/j.molcel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Gandhari MK, Frazier CR, Hartenstein JS, Cloix JF, Bernier M, Wainer IW. Identification and characterization of estrogen receptor-related receptor alpha and gamma in human glioma and astrocytoma cells. Molecular and cellular endocrinology. 2009 doi: 10.1016/j.mce.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 19.Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- 20.Holt PR. The roles of bile acids during the process of normal fat and cholesterol absorption. Arch Intern Med. 1972;130:574–583. [PubMed] [Google Scholar]
- 21.Kallen J, Schlaeppi JM, Bitsch F, Filipuzzi I, Schilb A, Riou V, Graham A, Strauss A, Geiser M, Fournier B. Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor alpha (ERRalpha): crystal structure of ERRalpha ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1alpha. The Journal of biological chemistry. 2004;279:49330–49337. doi: 10.1074/jbc.M407999200. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, Shigenaga J, Moser A, Grunfeld C, Feingold KR. Suppression of DHEA sulfotransferase (Sult2A1) during the acute-phase response. Am J Physiol Endocrinol Metab. 2004;287:E731–E738. doi: 10.1152/ajpendo.00130.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kim MS, Shigenaga J, Moser A, Grunfeld C, Feingold KR. Suppression of DHEA sulfotransferase (Sult2A1) during the acute-phase response. American journal of physiology. 2004;287:E731–E738. doi: 10.1152/ajpendo.00130.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kitada H, Miyata M, Nakamura T, Tozawa A, Honma W, Shimada M, Nagata K, Sinal CJ, Guo GL, Gonzalez FJ, Yamazoe Y. Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. J Biol Chem. 2003;278:17838–17844. doi: 10.1074/jbc.M210634200. [DOI] [PubMed] [Google Scholar]
- 25.Kitada H, Miyata M, Nakamura T, Tozawa A, Honma W, Shimada M, Nagata K, Sinal CJ, Guo GL, Gonzalez FJ, Yamazoe Y. Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. The Journal of biological chemistry. 2003;278:17838–17844. doi: 10.1074/jbc.M210634200. [DOI] [PubMed] [Google Scholar]
- 26.Kraus RJ, Ariazi EA, Farrell ML, Mertz JE. Estrogen-related receptor alpha 1 actively antagonizes estrogen receptor-regulated transcription in MCF-7 mammary cells. The Journal of biological chemistry. 2002;277:24826–24834. doi: 10.1074/jbc.M202952200. [DOI] [PubMed] [Google Scholar]
- 27.Noble JE, Bailey MJ. Quantitation of protein. Methods in Enzymology. 2009;463:73–95. doi: 10.1016/S0076-6879(09)63008-1. [DOI] [PubMed] [Google Scholar]
- 28.Ogura K, Satsukawa M, Okuda H, Hiratsuka A, Watabe T. Major hydroxysteroid sulfotransferase STa in rat liver cytosol may consist of two microheterogeneous subunits. Chemico-biological interactions. 1994;92:129–144. doi: 10.1016/0009-2797(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 29.Runge-Morris M, Kocarek TA. Regulation of sulfotransferases by xenobiotic receptors. Curr Drug Metab. 2005;6:299–307. doi: 10.2174/1389200054633871. [DOI] [PubMed] [Google Scholar]
- 30.Seely J, Amigh KS, Suzuki T, Mayhew B, Sasano H, Giguere V, Laganiere J, Carr BR, Rainey WE. Transcriptional regulation of dehydroepiandrosterone sulfotransferase (SULT2A1) by estrogen-related receptor alpha. Endocrinology. 2005;146:3605–3613. doi: 10.1210/en.2004-1619. [DOI] [PubMed] [Google Scholar]
- 31.Seo YK, Chung YT, Kim S, Echchgadda I, Song CS, Chatterjee B. Xenobiotic- and vitamin D-responsive induction of the steroid/bile acid-sulfotransferase Sult2A1 in young and old mice: the role of a gene enhancer in the liver chromatin. Gene. 2007;386:218–223. doi: 10.1016/j.gene.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonian MH, Smith JA. Spectrophotometric and colorimetric determination of protein concentration. Curr Protoc Mol Biol. 2006;Chapter 10(Unit 10):11A. doi: 10.1002/0471142727.mb1001as76. [DOI] [PubMed] [Google Scholar]
- 33.Sladek R, Bader JA, Giguere V. The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Molecular and cellular biology. 1997;17:5400–5409. doi: 10.1128/mcb.17.9.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stokes K, Alston-Mills B, Teng C. Estrogen response element and the promoter context of the human and mouse lactoferrin genes influence estrogen receptor alpha-mediated transactivation activity in mammary gland cells. Journal of molecular endocrinology. 2004;33:315–334. doi: 10.1677/jme.1.01456. [DOI] [PubMed] [Google Scholar]
- 35.Sumi D, Ignarro LJ. Estrogen-related receptor alpha 1 up-regulates endothelial nitric oxide synthase expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14451–14456. doi: 10.1073/pnas.2235590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun PM, Gao M, Wei LH, Mustea A, Wang JL, Konsgen D, Lichtenegger W, Sehouli J. An estrogen receptor alpha-dependent regulation of estrogen receptor-related receptor alpha in the proliferation of endometrial carcinoma cells. International journal of gynecological cancer. 2006;16 Suppl 2:564–568. doi: 10.1111/j.1525-1438.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 37.Sun PM, Wei LH, Sehouli J, Denkert C, Zhao D, Gao M, Sun XL, Litchtenegger W. [Role of estrogen receptor-related receptors alpha, beta and gamma in ovarian cancer cells] Zhonghua fu chan ke za zhi. 2005;40:544–548. [PubMed] [Google Scholar]
- 38.Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H. Estrogen-related receptor alpha in human breast carcinoma as a potent prognostic factor. Cancer research. 2004;64:4670–4676. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- 39.Thomae BA, Eckloff BW, Freimuth RR, Wieben ED, Weinshilboum RM. Human sulfotransferase SULT2A1 pharmacogenetics: genotype-to-phenotype studies. The pharmacogenomics journal. 2002;2:48–56. doi: 10.1038/sj.tpj.6500089. [DOI] [PubMed] [Google Scholar]
- 40.Vanacker JM, Delmarre C, Guo X, Laudet V. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related alpha. Cell growth & differentiation. 1998;9:1007–1014. [PubMed] [Google Scholar]
- 41.Vanacker JM, Pettersson K, Gustafsson JA, Laudet V. Transcriptional targets shared by estrogen receptor-related receptors (ERRs) and estrogen receptor (ER) alpha, but not by ERbeta. The EMBO journal. 1999;18:4270–4279. doi: 10.1093/emboj/18.15.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vargas SO, Leslie KO, Vacek PM, Socinski MA, Weaver DL. Estrogen-receptor-related protein p29 in primary nonsmall cell lung carcinoma: pathologic and prognostic correlations. Cancer. 1998;82:1495–1500. [PubMed] [Google Scholar]
- 43.Watanabe A, Kinoshita Y, Hosokawa K, Mori T, Yamaguchi T, Honjo H. Function of estrogen-related receptor alpha in human endometrial cancer. The Journal of clinical endocrinology and metabolism. 2006;91:1573–1577. doi: 10.1210/jc.2005-1990. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 45.Yanagiba Y, Ito Y, Kamijima M, Gonzalez FJ, Nakajima T. Octachlorostyrene induces cytochrome P450, UDP-glucuronosyltransferase, and sulfotransferase via the aryl hydrocarbon receptor and constitutive androstane receptor. Toxicol Sci. 2009;111:19–26. doi: 10.1093/toxsci/kfp130. [DOI] [PubMed] [Google Scholar]
- 46.Yang N, Shigeta H, Shi H, Teng CT. Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. The Journal of biological chemistry. 1996;271:5795–5804. doi: 10.1074/jbc.271.10.5795. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Teng CT. Estrogen receptor alpha and estrogen receptor-related receptor alpha1 compete for binding and coactivator. Molecular and cellular endocrinology. 2001;172:223–233. doi: 10.1016/s0303-7207(00)00372-5. [DOI] [PubMed] [Google Scholar]
- 48.Zirngibl RA, Chan JS, Aubin JE. Estrogen receptor-related receptor alpha (ERRalpha) regulates osteopontin expression through a non-canonical ERRalpha response element in a cell context-dependent manner. Journal of molecular endocrinology. 2008;40:61–73. doi: 10.1677/JME-07-0114. [DOI] [PubMed] [Google Scholar]