Abstract
The set size effect in visual search refers to the linear increase in response time (RT) or decrease in accuracy as the number of distractors increases. Previous human and monkey studies have reported a correlation between set size and neural activity in the frontal eye field (FEF) and intraparietal sulcus (IPS). In a recent functional magnetic resonance imaging (fMRI) study, we did not observe a set size effect in the superior precentral sulcus (sPCS, thought to be the human homologue of the FEF) and IPS in an oculomotor visual search task (Ikkai et al., in press). Our task used placeholders in the search array, along with the target and distractors, in order to equate the amount of retinal stimulation for each set size. We here attempt to reconcile these differences with results from a follow-up experiment in which the same oculomotor visual search task was used, but without placeholders. A strong behavioral set size effect was observed in both studies, with very similar saccadic RTs and slopes between RT and set size. However, a set size effect was now observed in sPCS and IPS. We comment on this finding and discuss the role of these neural areas in visual search.
Keywords: visual search, set size, parietal cortex, frontal cortex, functional magnetic resonance imaging, saccade
Introduction
The visual world is a cornucopia of clutter from which the attention system must select relevant information. For decades, visual search tasks have been used to explore the principles and mechanisms of visual perception and visual attention (Treisman & Gelade, 1980; Chelazzi, 1999; Wolfe & Horowitz, 2004). In a typical visual search task, subjects detect a target that is embedded within an array of distractors. Increasing the number of distractors tends to produce a higher RT and lower accuracy, a finding known as the set size effect (Carrasco & Yeshurun, 1998; Wolfe et al., 1998; McElree & Carrasco, 1999). The set size effect is associated with automatic “pre-attentive” processing, various attentional mechanisms, and conscious search for the target (Treisman, 1991; Wolfe & Horowitz, 2004).
Research on the neural basis of the set size effect is challenging because a change in set size may modify the perceptual and cognitive demands of a task. For example, a change in set size may vary the amount of visual information in the display, thereby altering brain activity in low-level visual areas; or it may differentially engage attentional areas, such as the posterior parietal cortex (PPC) (Robinson et al., 1995; Corbetta & Shulman, 2002; Jerde et al., 2008); or it may vary the number of potential targets for attention and action, thereby changing the demands on target selection; and so on. It is thus not surprising that neuroimaging studies have reported a set size effect in different brain regions (e.g., occipital, parietal, and prefrontal cortices) (Leonards et al., 2000; Muller et al., 2003; Anderson et al., 2007). In monkey electrophysiological studies, the firing rate of neurons in the frontal eye field (FEF) (Cohen et al., 2009a) and the lateral intraparietal area (LIP) (Balan et al., 2008) declines as set size increases. This finding is thought to reflect an increase in competitive interactions among neurons for which the potential targets lie in their receptive fields (Kastner et al., 2001; Schall et al., 2004; Cohen et al., 2010).
Using rapid event-related fMRI, we recently reported that regions in prefrontal cortex (PFC) and PPC do not show a set size effect in an oculomotor visual search task (Ikkai et al., in press), a finding that seems at odds with other studies (Balan et al., 2008; Cohen et al., 2009a). Importantly, our saccadic response time (SRT) data showed a robust set size effect, namely a linear increase in SRT as the target-to-distractor ratio increased (Fig. 2a). Furthermore, we observed a robust set size effect in occipital and temporal regions, indicating that our experiment taxed perceptual processing; moreover, SRT correlated with BOLD activity in occipital, parietal, and prefrontal cortices (Fig. 3a), indicating that neural activation reflected the demands of the task. One key difference between the visual search task used in Ikkai et al. (in press) and those used in other studies was the use of placeholders in the stimulus array (Fig. 1a). Our rationale for using placeholders was to avoid the potential confounds of manipulating set size by merely changing the number of distractors and not controlling for retinal stimulation, as mentioned above.
Figure 2.
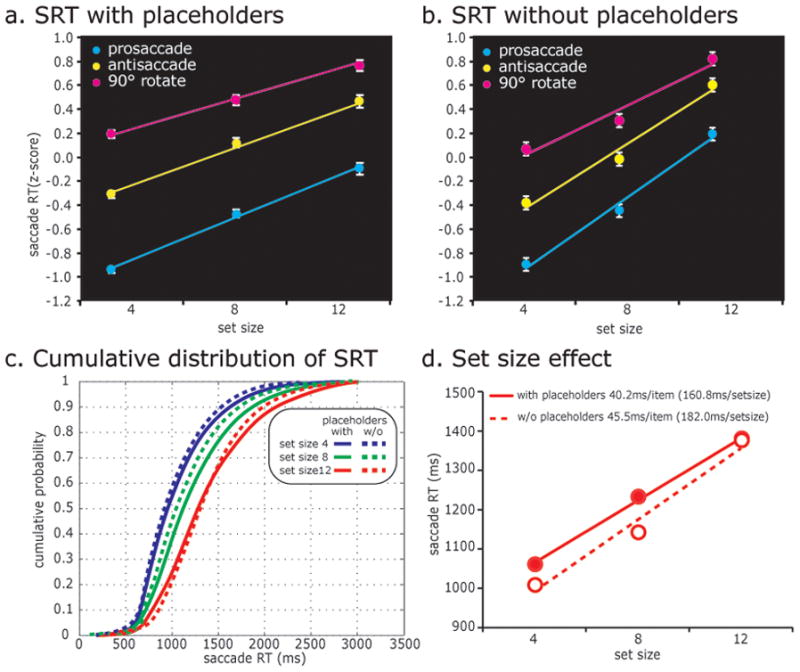
Behavioral data. The average saccadic response time (SRT) data across all subjects in (a) the placeholder (N=18) and (b) no placeholder (N=4) conditions. Each subject’s SRT was converted into z-scores before averaging. Error bars are SEM. Note the positive linear increase in SRT as a function of set size and saccade transformation. (c) Cumulative distributions of SRT for each set size. The search array comes on at t = 0. Solid lines indicate SRT from the experiment with placeholders and dotted lines indicate SRT from the experiment without placeholders. SRTs for smaller set sizes are faster and the distributions for each set size are similar regardless of the use of placeholders. (d) Set size effect on SRT can be discerned from the positive slopes. Set size slopes are similar with placeholders (solid) and without them (dashed).
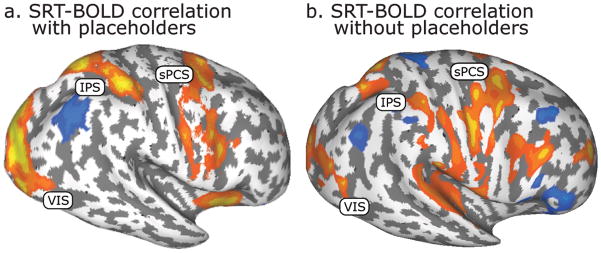
Figure 3.
Cortical regions in which BOLD activity correlated with SRT (a) with placeholders (N=18) and (b) without them (N=4). Warm colors show that the region’s BOLD signal increased as the SRT increased, whereas cool colors show that the BOLD signal decreased as the SRT increased.
Figure 1.
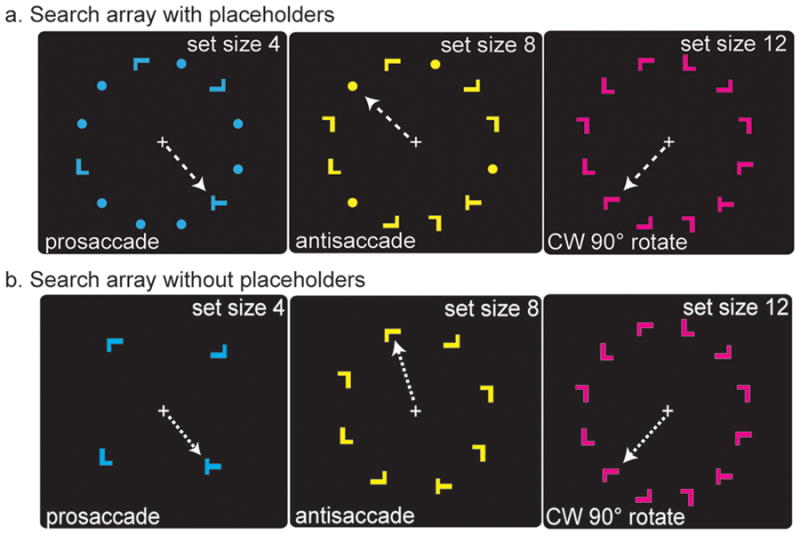
Visuomotor search task, example trials. Subjects fixated a white cross during a variable intertrial interval (3~11 sec) that served as a baseline. Upon presentation of the search array, subjects covertly searched for the letter “T” among letter “L” distractors. The left, middle, and right panels show examples of trials in which there were 4, 8, and 12 item search arrays. The left, middle, and right panels show examples of trials in which subjects looked towards the target (prosaccade), away from the target (antisaccade), or rotated 90° clockwise from the target. The color of the search array indicated which saccade transformation to apply. Although the color-transformation assignment was counterbalanced across subjects, in this example, cyan, yellow, and magenta instructed a prosaccade, antisaccade, or rotation saccade, respectively (indicated by the white dotted arrow, invisible to subjects). Two versions of the task are depicted, one with placeholders that equate the amount of retinal stimulation (a) as used in Ikkai et al. (in press), and one without placeholders (b) used in the present study.
However, the use of placeholders may also have equated the number of potential targets across set sizes. That is, despite the clear existence of a set size effect at the behavior level and in extrastriate visual cortex, the presence of placeholders meant that 12 potential targets were present for each set size (Fig. 1a). We hypothesized that this search array might have been responsible for the lack of a set size effect in PFC and PPC reported in Ikkai et al. (in press). In the present follow-up experiment, we modified the search array by removing the placeholders and scanned subjects using the same experimental and statistical procedures as in Ikkai et al. (in press). Set size 4 now contained only four items, set size 8 contained only eight items, and set size 12 contained twelve items (Fig. 1b); thus the number of distractors as well as the amount of retinal stimulation varied across set sizes. We then reexamined whether activation in sPCS and IPS was related to set size.
Methods
Aside from the lack of placeholders used here, the visual search task, imaging procedures, and analyses are essentially identical to those used in our previous study (Ikkai et al., in press). Four neurologically healthy subjects (three males, all right-handed, aged between 24–40 years) were recruited for participation and paid for their time. Subjects gave written informed consent and all procedures were in compliance with the safety guidelines for fMRI research and approved by the human subjects Institutional Review Board at New York University.
Behavioral procedures and factorial design
The experimental stimuli were controlled by E-Prime (Psychology Software Tools, Inc., Pittsburgh, PA) and projected (Eiki LC-XG100) into the bore of the scanner on a screen that was viewed by the subjects through an angled mirror. Subjects fixated a central white cross against a black background until a search array was presented. Examples of three of the nine possible search array displays are shown in Fig. 1b. Search arrays consisted of one target (letter “T”) and 3, 7, or 11 distractors (letter “L). Both stimuli were 0.64 degrees of visual angle high and wide, and presented within an invisible annulus with an outer radius of 5.75 degrees of visual angle. Search arrays were visible for 3 sec, while subjects covertly searched for the target (i.e., gaze remained at fixation). Target and distractors could be presented in any of 3 colors (yellow, magenta, and cyan), and in any of 4 orientations (0, 90, 180, and 270 degrees). A variable inter-trial interval (ITI: 3, 5, 7, 9, or 11 sec) was used between search trials.
A fully crossed factorial design with two factors, set size and saccade transformation, each with 3 levels, yielded a total of nine trial types. To manipulate set size, search arrays consisted of 4, 8, or 12 letter items. To manipulate saccade transformation, the color of the search array specified the saccade transformation (Fig. 1): the color instructed subjects to make a saccade to the target (prosaccade), 180 degrees opposite from the target (antisaccade), or rotated clockwise or counterclockwise 90 degrees from the target (rotated saccade). The color-saccade transformation assignment was counterbalanced across subjects and the order of trial types was pseudo-randomized. The location of the target and the color of the search array were pseudo-randomized so that neither the search nor saccade target appeared in the same place on more than two trials in a row, and the same color did not repeat on more than two trials. Each scanning session consisted of 8 blocks of four trials per condition, yielding a total of 36 trials per block and a total of 32 trials per condition in a scanning session.
It is important to note that the factorial design allowed us to directly assess whether an interaction existed between the two factors, namely set size and saccade transformation. For set size, the three levels were 4, 8, or 12 items in the search array; for saccade transformation, the three levels were prosaccade antisaccade, and rotated saccade. An interaction between set size and saccade transformation would occur if the differences on one factor depended on the level of the other factor. Critically, the two factors did not interact at the behavioral and neural levels, as measured in the statistical analyses (see Results). The lack of an interaction meant that the effects of set size could be meaningfully assessed independent of the levels of saccade transformation.
Oculomotor Procedures
Eye position was monitored in the scanner at 60 Hz with an infrared videographic camera equipped with a telephoto lens (ASL 504LRO; Applied Sciences Laboratories, Bedford, MA; modified with a Sony HAD CCD) that focused on the right eye viewed from the flat surface mirror mounted inside the RF coil. Nine-point calibrations were performed at the beginning of the session and between blocks when necessary. Eye-movement data were transformed to degrees of visual angle, calibrated using a third-order polynomial algorithm that fit eye positions to known spatial positions, and scored offline with in-house software (GRAPES). Any trials with unwanted/incorrect saccades were discarded (e.g. overt search, corrective saccade, saccade to wrong item). Only trials in which the first saccade landed on the correct target and remained there until the search array offset were further analyzed. Saccadic reaction times were estimated with semiautomatic routines that relied on the velocity of the eye reaching about 30°/s to determine the onset of saccades. The data were also inspected visually, trial by trial, and corrections were made if necessary.
fMRI Procedures
fMRI data were collected using a 3T head-only scanner (Allegra, Siemens, Germany) at the Center for Brain Imaging at New York University. Images were acquired using custom radio frequency coils (NM-011 transmit head-coil and NMSC-021 four-channel phased array receive coil; NOVA Medical, Wakefield, MA) placed over lateral frontal and parietal cortices. During each fMRI scan, a series of volumes was acquired using a T2*-sensitive echo planar imaging pulse sequence (repetition time, 2000 ms; echo time, 30 ms; flip angle, 80°; 36 slices; 3 × 3 × 3 mm voxels; 192 × 192 mm FOV). High-resolution (1 mm isotropic voxels) MP-RAGE three-dimensional T1-weighted scans were acquired for anatomical registration, segmentation, and display.
BOLD Analytic Procedures
Post hoc image registration was used to correct for residual head motion [MCFLIRT (motion correction using the Linear Image Registration Tool from Oxford University’s Center for Functional MRI of the Brain)] (Jenkinson et al., 2002). Additional preprocessing of the fMRI data was as follows. First, the time series of each voxel was band-passed (0.05 to 0.25 Hz) to compensate for the slow drift typical in fMRI measurements (Zarahn et al., 1997), and divided by its mean intensity to convert to percent signal modulation and compensate for the decrease in mean image intensity with distance from the receive coil. The fMRI response was modeled with an impulse time-locked to the onset of the search array convolved with a canonical hemodynamic response function (HRF) (Polonsky et al., 2000). Each level of both factors (e.g., set size 4-prosaccade, set size 4-antisaccade, …, set size 12-rotated saccade, etc.) were modeled separately in the design matrix and entered into a modified general linear model (GLM (Worsley & Friston, 1995)) for statistical analysis using VoxBo (http://www.voxbo.org). For each subject, Caret (http://brainmap.wustl.edu/caret) was used for anatomical segmentation, gray-white matter surface generation, flattening, and multi-fiducial deformation mapping to the PALS atlas (Van Essen, 2005). To examine the relationship between RT and the BOLD signal, statistical maps were computed that reflected correlations between evoked BOLD activity and SRT on a trial-by-trial basis. To do so, excluding incorrect trials, RT (convolved with a HRF) was regressed against BOLD time-courses.
Time-series analytic procedures
Region of interest (ROI) based analyses were used to examine the time courses of BOLD signal change. First, on each subject’s high-resolution anatomical scans, the grey matter was traced along ROIs. ROIs included sPCS (along the precentral sulcus and lateral to the junction with the superior frontal sulcus); IPS (from the junction with the postcentral sulcus to the junction with the parieto-occipital sulcus); and an extrastriate region along the collateral sulcus here denoted VIS that showed a set size effect in Ikkai et al. (in press) and Leonards et al. (2000). Within each ROI, an F-test was used to select 20 voxels (540 mm3) with the strongest overall task effect; these voxels showed a consistent deviation from baseline during the task. The selection is unbiased by activation (could be negative or positive relative to baseline) or trial type (none of the 2 factors × 3 levels are given unique weight). BOLD data were converted into percent signal change, and time-courses time-locked to the onset of the search array were deconvolved using AFNI (http://afni.nimh.nih.gov/afni), with no hemodynamic response assumed. The estimated impulse response functions were averaged across voxels within an ROI and averaged across subjects from analogous ROIs to visualize time-series. Error bars are standard deviations between subjects at each time point. For an individual subject, the average of 3 TRs around the peak of the impulse response function (time points 4, 6, and 8 sec) from each condition was extracted from each ROI and used as a dependent variable in statistical analyses of the time-courses.
Results
Behavioral Results
Remarkably, the behavioral dependent measures were almost identical regardless of whether placeholders were used or not. Across trial types, subjects were on average 82% accurate without placeholders, compared to 86% accurate with them (Ikkai et al., in press). A repeated measures ANOVA of accuracy revealed a marginally significant effect of set size (F2,6 = 4.77, P = 0.06), but no significant effect for saccade transformation (F2,6 = 0.79, P = 0.50), and the interaction between the two was not significant (F4,12 = 0.17, P = 0.95). A repeated measures ANOVA of SRT revealed a significant effect of set size (F2,6 = 48.90, P = 0.00019) and saccade transformation (F2,6 = 8.0, P = 0.02), and the interaction between the two was not significant (F4,12 = 1.3, P = 0.31). As predicted, subject performance was better when the set size was smaller, and when the saccade transformation was simpler (Table 1). Fig. 2a/b shows the average SRT of all subjects. The slope of the set size effect was very similar regardless of placeholders (45.5 msec/item with placeholders versus 40.2 msec/item without placeholders) (Fig. 2d). The magnitude of this set size effect is on par with that found in behavioral studies (Carrasco & Yeshurun, 1998; Horowitz & Wolfe, 1998; Wolfe, 2010). The results for set size 12 were identical in the two studies because no placeholders were used in either version; accordingly, as one would predict, the SRTs were identical. Overall, behaviorally, the only difference between the two versions was a slightly steeper slope (5.3 msec/item) when placeholders were not used (Fig. 2d). One can appreciate this by comparing the slightly shifted cumulative distributions shown in Fig. 2c. Importantly, since the two main effects (set size and saccade transformation) did not interact, it is straight forward to interpret the effects of increasing the set size.
Table 1.
Behavioral data
| set size | prosacade | antisacade | rotated sacade | mean | |
|---|---|---|---|---|---|
| accuracy (%) | 4 | 92.6 (7.6) | 91.1 (6.5) | 88.7 (3.8) | 90.8 |
| accuracy (%) | 8 | 89.6 (10.0) | 76.8 (15.9) | 77.8 (21.0) | 81.4 |
| accuracy (%) | 12 | 78.8 (10.9) | 70.6 (5.1) | 74.3 (17.8) | 74.6 |
| accuracy (%) | mean | 87.0 (7.3) | 79.5 (10.5) | 80.3 (7.5) | 82.3 |
| RT (ms) | 4 | 799.4 (212.1) | 1021.1 (315.7) | 1227.7 (407.8) | 1012.1 (364.6) |
| RT (ms) | 8 | 970.2 (345.5) | 1147.9 (385.8) | 1297.4 (422.4) | 1133.3 (406.5) |
| RT (ms) | 12 | 1225.8 (441.9) | 1414.9 (461.2) | 1494.7 (450.4) | 1376.2 (463.6) |
| RT (ms) | mean | 985.0 (380.2) | 1180.2 (418.1) | 1332.9 (439.2) | 1162.3 (436.5) |
Values are means (SD); N = 4 subjects.
Imaging Results: Correlation of BOLD signal with Reaction Time
As in Ikkai et al. (in press), we here replicate positive correlations between behavioral SRTs and bilateral activation in sPCS and IPS, as well as widespread occipital cortex (Fig. 3), indicating a strong coupling of neural activation with task performance. Positive correlations may reflect the greater neural activity (duration or magnitude) associated with the more demanding level of the factor (e.g., set size: set size 12 > set size 8 > set size 4) that resulted in longer SRTs.
Imaging Results: ROI time-series analyses
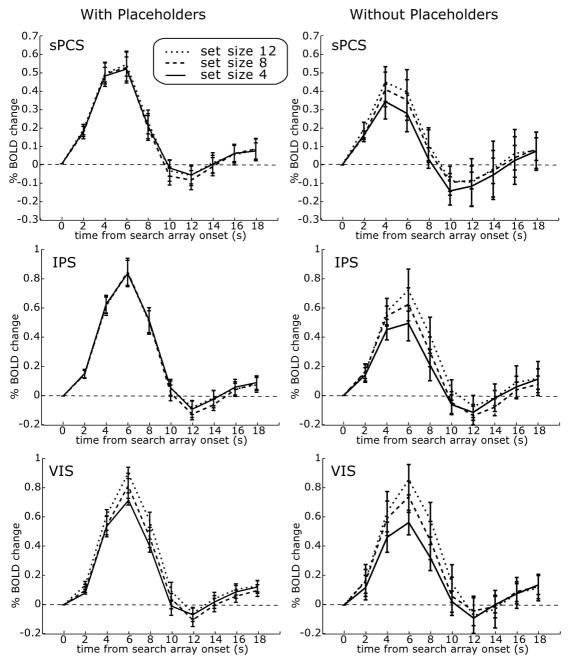
Recall that when placeholders were used in Ikkai et al. (in press), BOLD activity in sPCS and IPS did not correlate with set size; rather, it only correlated with the saccade transformation (Fig. 4, left). Using these same procedures, we plot the subject-averaged time series from ROIs time-locked to the onset of the search array. The BOLD activity in all ROIs did not show a significant interaction between set size and saccade transformation in either study, again simplifying the interpretation of the main effect of set size. The IPS, sPCS, and VIS ROIs showed a significant or nearly significant linear increase in the BOLD signal, and strong effect sizes, as the set size increased: right and left IPS combined, F1,3 = 13.23, P = 0.036, effect size = 0.82; left IPS: F1,3 = 13.69, P = 0.03, effect size = 0.82; right IPS: F1,3 = 9.40, P = 0.05, effect size = 0.76; left and right sPCS combined: F1,3 = 8.98, P = 0.06, effect size = 0.75; left sPCS: F1,3 = 12.02, P = 0.04, effect size = 0.80; right sPCS: F1,3 = 2.99, P = 0.18, effect size = 0.50; left and right VIS combined: F1,3 = 31.53, P = 0.01, effect size = 0.91; left VIS: F1,3 = 40.74, P = 0.01, effect size = 0.93; right VIS: F1,3 = 15.26, P = 0.03, effect size = 0.84. In summary, sPCS, IPS, and VIS correlate with overall SRT in both task versions. sPCS and IPS only show a set size effect when placeholders are not used. Finally, VIS shows a set size effect regardless of whether placeholders were used or not.
Figure 4.
Deconvolved time-series from the search task with placeholders (left, N=18) and without placeholders (right, N=4). The search array came on at t = 0. The plots represent means ±1 SD across subjects. When placeholders are used, only portions of visual cortex showed an effect of set size. When placeholders were not used, sPCS and IPS showed an effect of set size. A set size effect was defined as an area that showed a linear increase in BOLD activity with an increase in set size.
Discussion
We here report that even when behavior was essentially indistinguishable, BOLD activity in sPCS and IPS differed remarkably during visual search depending on whether placeholders were used to equate for retinal stimulation. It would be easy to disregard these effects if the behavior were not so similar. Moreover, overall SRTs correlated with BOLD in sPCS and IPS regardless of whether placeholders were used or not. Therefore, the lack of a set size effect in frontal and parietal cortices when placeholders were used (Ikkai et al., in press) cannot be attributed to either a restriction in the range of SRTs for the placeholder condition (Fig. 2) or a lack of association with behavior (Fig. 3). So how do we make sense of these findings?
First, when no placeholders were used in the current study, the results could simply be due to the increased amount of retinal stimulation with increasing set sizes. Indeed, both sPCS and IPS contain neurons that increase in activity when irrelevant stimuli are placed within their receptive fields (Mohler et al., 1973; Bisley et al., 2004; Ipata et al., 2009). Second, the use of placeholders (Ikkai et al., in press) not only equated retinal stimulation, but to the sPCS and IPS, it may have equated the number of potential saccade goals across set sizes. That is, by using placeholders, set sizes 4, 8, and 12 all had the same number of potential saccade goals, namely 12 (Fig. 1a). Thus, one would not necessarily expect to find a set size effect in brain areas that select among saccade goals, such as FEF and LIP (Schall & Hanes, 1993; Bichot & Schall, 2002; Ipata et al., 2006; Thomas & Pare, 2007; Ipata et al., 2009), because the number of potential saccade goals was identical across set sizes when placeholders were used. Third, the brain as a whole may not have treated the placeholders used in Ikkai et al. (in press) as equivalent to the L distractors, since a strong set size effect was observed at the behavioral level (Fig. 2) and in extrastriate visual cortex (Fig. 4). Target identification during visual search is based on feature discrimination, which likely depended on computations performed in extrastriate cortex (Gregoriou et al., 2009a), not human homologues of monkey areas FEF or LIP that do not seem to have feature selectivity.
Recent monkey electrophysiology studies have examined the role of FEF (Cohen et al., 2009a) and LIP (Balan et al., 2008) on the set size effect during visual search. As set size increased, monkeys took more time to find the target, and the peak firing rate of neurons in both FEF and LIP decreased in proportion to the longer RT. The most likely mechanism of this effect is the interplay between competitive and cooperative interactions among neurons in locating the search target (Schall et al., 2004; Cohen et al., 2010). Interestingly, the target selection time (TST), which is the time when a neuron’s activity distinguishes between when the target is in its receptive field (RF) versus when a distractor is in the RF (Cohen et al., 2009a), was reported to increase in FEF as the set size increased (Cohen et al., 2009a), but not in LIP (Balan et al., 2008). The relation of TST to set size is a matter of ongoing discussion (Balan & Gottlieb, 2009; Cohen et al., 2009b). We speculate that if those researchers had used placeholders, the TSTs across set size may have collapsed or at the very least narrowed and delayed. One important clarification that should be made here is that target selection in this case does not refer to identifying the target’s identity, per se. Instead, it refers to identifying the location of the target. FEF and LIP neurons have poor feature selectivity and could not distinguish between a T and L in their RFs. Therefore, the competitive interactions that lead to spatial selection are among neurons coding for different visual field locations that contain targets and distractors.
In our experiment without placeholders, sPCS and IPS showed increasing BOLD activity with increasing set size. Since fMRI best characterizes the population of neural activity in an area, this linear increase may reflect a greater number of neurons in active competition as the set size increases (Balan et al., 2008; Cohen et al., 2009a). Theoretically, the ongoing activity in sPCS and IPS may form maps of prioritized space (Thompson & Bichot, 2005; Fecteau & Munoz, 2006; Gottlieb, 2007; Bisley & Goldberg, 2010). The locations of relevant stimuli and saccade goals may be represented in spatial topographic maps by the activation levels of neurons with RFs that contain the targets and distractors. If these maps are indeed agnostic to the features of stimuli whose locations are prioritized, this may explain why we failed to find a set size effect when placeholders were used. So what leads to spatial selection in FEF and LIP? Consider that extrastriate cortical areas (e.g., V4) are able to discriminate between the visual features in the stimulus array (Chelazzi et al., 2001; Bichot et al., 2005), and we found correlations between BOLD activity and set size regardless of the use of placeholders. The output of these selective processes for visual features could bias activation in the frontal and parietal priority maps in favor of neurons with RFs that include the target. Visual search would then reflect the ongoing interactions between top-down inputs about the prioritized locations from areas such as FEF and LIP to visual areas such as V4, and bottom-up inputs from V4 to FEF as selective processing for visual features is used to identify the search target (Gregoriou et al., 2009a; b).
We caution that our oculomotor search task is not a pure visual search task, since it involved a saccade transformation, in aid of goals unrelated to the current discussion. Thus, our task is a hybrid of classic visual search tasks, with their demands on visual perception and visual attention, and response selection tasks, in which actions are selected among competing alternatives (Teichner & Krebs, 1974). Indeed, an interesting parallel can be drawn between the set size effect in visual search and Hick’s law, which states that RT increases as a function of the number of response alternatives (Hick, 1952). Saccadic eye movements follow Hick’s law (Lee et al., 2005), and FEF (Lee & Keller, 2008) and IPS (Lee et al., 2006) neurons are modulated by the number of alternatives in response selection. Given that perception and action are integrated in natural behavior, search tasks like the one described here could be useful for investigating the neural basis of attention and action.
Acknowledgments
We thank the Center for Brain Imaging at NYU and their staff for support during data collection. This work was supported by NIH R01 EY016407 to CEC and by NIH NRSA F32 EY019221 to TAJ.
Footnotes
The authors declare no conflict of interest.
References
- Anderson EJ, Mannan SK, Husain M, Rees G, Sumner P, Mort DJ, McRobbie D, Kennard C. Involvement of prefrontal cortex in visual search. Exp Brain Res. 2007;180:289–302. doi: 10.1007/s00221-007-0860-0. [DOI] [PubMed] [Google Scholar]
- Balan P, Gottlieb J. Comment on Cohen et al. Neural Basis of the Set-Size Effect in Frontal Eye Field: Timing of Attention During Visual Search. J Neurophysiol. 2009;102:1340–1341. doi: 10.1152/jn.00302.2009. author reply 1342–1343. [DOI] [PubMed] [Google Scholar]
- Balan PF, Oristaglio J, Schneider DM, Gottlieb J. Neuronal correlates of the set-size effect in monkey lateral intraparietal area. PLoS Biol. 2008;6:e158. doi: 10.1371/journal.pbio.0060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. doi: 10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Priming in macaque frontal cortex during popout visual search: feature-based facilitation and location-based inhibition of return. J Neurosci. 2002;22:4675–4685. doi: 10.1523/JNEUROSCI.22-11-04675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2004;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Yeshurun Y. The contribution of covert attention to the set-size and eccentricity effects in visual search. J Exp Psychol Hum Percept Perform. 1998;24:673–692. doi: 10.1037//0096-1523.24.2.673. [DOI] [PubMed] [Google Scholar]
- Chelazzi L. Serial attention mechanisms in visual search: a critical look at the evidence. Psychol Res. 1999;62:195–219. doi: 10.1007/s004260050051. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. Responses of neurons in macaque area V4 during memory-guided visual search. Cereb Cortex. 2001;11:761–772. doi: 10.1093/cercor/11.8.761. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Crowder EA, Heitz RP, Subraveti CR, Thompson KG, Woodman GF, Schall JD. Cooperation and competition among frontal eye field neurons during visual target selection. J Neurosci. 2010;30:3227–3238. doi: 10.1523/JNEUROSCI.4600-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Heitz RP, Woodman GF, Schall JD. Neural basis of the set-size effect in frontal eye field: timing of attention during visual search. J Neurophysiol. 2009a;101:1699–1704. doi: 10.1152/jn.00035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Heitz RP, Woodman GF, Schall JD. Reply to Balan and Gottlieb. J Neurophysiol. 2009b;102:1342–1343. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009a;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. Long-range neural coupling through synchronization with attention. Prog Brain Res. 2009b;176:35–45. doi: 10.1016/S0079-6123(09)17603-3. [DOI] [PubMed] [Google Scholar]
- Hick WE. On the rate of gain of information. Quart J Exp Psychol. 1952;4:11–26. [Google Scholar]
- Horowitz TS, Wolfe JM. Visual search has no memory. Nature. 1998;394:575–577. doi: 10.1038/29068. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Jerde TA, Curtis CE. Perception and action selection dissociate human ventral and dorsal cortex. J Cogn Neurosci. doi: 10.1162/jocn.2010.21499. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Bisley JW, Goldberg ME. Neurons in the lateral intraparietal area create a priority map by the combination of disparate signals. Exp Brain Res. 2009;192:479–488. doi: 10.1007/s00221-008-1557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerde TA, Lewis SM, Goerke U, Gourtzelidis P, Tzagarakis C, Lynch J, Moeller S, Van de Moortele PF, Adriany G, Trangle J, Ugurbil K, Georgopoulos AP. Ultra-high field parallel imaging of the superior parietal lobule during mental maze solving. Exp Brain Res. 2008;187:551–561. doi: 10.1007/s00221-008-1318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Pinsk MA, Elizondo MI, Desimone R, Ungerleider LG. Modulation of sensory suppression: implications for receptive field sizes in the human visual cortex. J Neurophysiol. 2001;86:1398–1411. doi: 10.1152/jn.2001.86.3.1398. [DOI] [PubMed] [Google Scholar]
- Lee KM, Keller EL. Neural activity in the frontal eye fields modulated by the number of alternatives in target choice. J Neurosci. 2008;28:2242–2251. doi: 10.1523/JNEUROSCI.3596-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Keller EL, Heinen SJ. Properties of saccades generated as a choice response. Exp Brain Res. 2005;162:278–286. doi: 10.1007/s00221-004-2186-5. [DOI] [PubMed] [Google Scholar]
- Lee KM, Wade AR, Lee BT. Differential correlation of frontal and parietal activity with the number of alternatives for cued choice saccades. Neuroimage. 2006;33:307–315. doi: 10.1016/j.neuroimage.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Leonards U, Sunaert S, Van Hecke P, Orban GA. Attention mechanisms in visual search -- an fMRI study. J Cogn Neurosci. 2000;12(Suppl 2):61–75. doi: 10.1162/089892900564073. [DOI] [PubMed] [Google Scholar]
- McElree B, Carrasco M. The temporal dynamics of visual search: evidence for parallel processing in feature and conjunction searches. J Exp Psychol Hum Percept Perform. 1999;25:1517–1539. doi: 10.1037//0096-1523.25.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler CW, Goldberg ME, Wurtz RH. Visual receptive fields of frontal eye field neurons. Brain Res. 1973;61:385–389. doi: 10.1016/0006-8993(73)90543-x. [DOI] [PubMed] [Google Scholar]
- Muller NG, Donner TH, Bartelt OA, Brandt SA, Villringer A, Kleinschmidt A. The functional neuroanatomy of visual conjunction search: a parametric fMRI study. Neuroimage. 2003;20:1578–1590. doi: 10.1016/s1053-8119(03)00416-6. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nature Neuroscience. 2000;3(11):1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Bowman EM, Kertzman C. Covert orienting of attention in macaques. II. Contributions of parietal cortex. J Neurophysiol. 1995;74:698–712. doi: 10.1152/jn.1995.74.2.698. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- Schall JD, Sato TR, Thompson KG, Vaughn AA, Juan CH. Effects of search efficiency on surround suppression during visual selection in frontal eye field. J Neurophysiol. 2004;91:2765–2769. doi: 10.1152/jn.00780.2003. [DOI] [PubMed] [Google Scholar]
- Teichner WH, Krebs MJ. Laws of visual choice reaction time. Psychol Rev. 1974;81:75–98. doi: 10.1037/h0035867. [DOI] [PubMed] [Google Scholar]
- Thomas NW, Pare M. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J Neurophysiol. 2007;97:942–947. doi: 10.1152/jn.00413.2006. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Treisman A. Search, similarity, and integration of features between and within dimensions. J Exp Psychol Hum Percept Perform. 1991;17:652–676. doi: 10.1037//0096-1523.17.3.652. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual search. Curr Biol. 2010;20:R346–R349. doi: 10.1016/j.cub.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM, Horowitz TS. What attributes guide the deployment of visual attention and how do they do it? Nat Rev Neurosci. 2004;5:495–501. doi: 10.1038/nrn1411. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, O’Neill P, Bennett SC. Why are there eccentricity effects in visual search? Visual and attentional hypotheses. Percept Psychophys. 1998;60:140–156. doi: 10.3758/bf03211924. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6(2):122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]