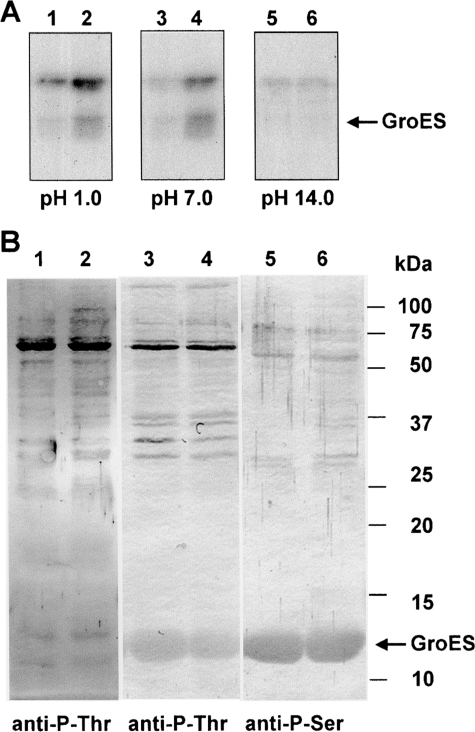
Figure 5.
Stability of phosphorylated GroES at different pHs. (A) Autoradiographs of the phosphorylated GroES. Purified GroES (2.5 μg) mixed with either protein extracts derived from control (32°C) wild-type cells (10 μg) (lanes 1, 3, and 5) or with heat-treated (44°C for 30 min) cells (10 μg) (lanes 2, 4, and 6) was phosphorylated in vitro with [γ-32P]ATP. The reaction was terminated with 3× concentrated SDS–PAGE sample buffer and immediately subjected to SDS–PAGE (15% PAG). After electrophoresis, the proteins were transferred onto PVDF membrane. The membranes were stained with Ponceau red and incubated at 45°C for 2 h in 50 mM KCl–HCl (pH 1.0), 0.1 M Tris–HCl (pH 7), or 1 M KOH (pH 14). The radioactivity remaining in the membrane was revealed after its exposure onto a X-ray film. (B). Immunoblots of proteins from the wild-type cells of Synechocystis probed with monoclonal antibodies against phosphorylated Ser and Thr (anit-P-Thr and anti-P-Ser). Left panel: Protein extracts (25 μg) isolated from control (lane 1) and heat-treated (lane 2) wild-type cells were probed with anti-P-Thr antibodies. Central panel: Protein extracts (25 μg) isolated from control (lanes 3 and 5) and heat-treated (lanes 4 and 6) wild-type cells were probed with anti-P-Thr antibodies after phosphorylation in vitro with exogenously added recombinant GroES (2.5 μg). Right panel: The same as the middle panel but probed with anti-P-Ser antibodies.