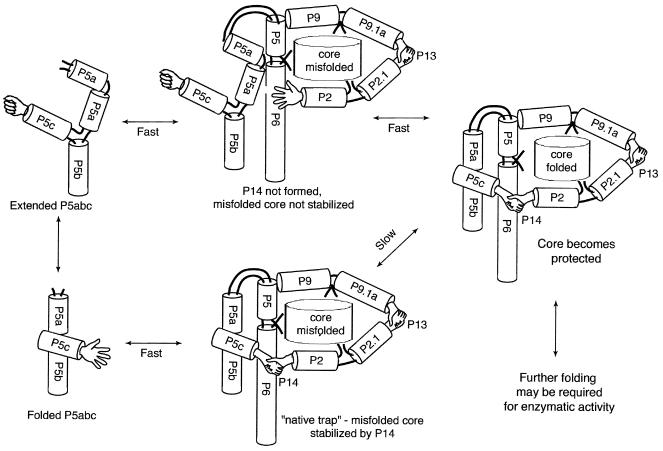
Figure 5.
A folding model of the group I intron ribozyme and a possible role of the GNRA tetraloop in an extended P5abc. Interdomain interactions P14 and P13, represented by two pairs of interacting hands, can stabilize the misfolded core (18). P14 is an interaction of the hands from L5c and L2. When all hands in the peripheral helices are closed, the catalytic core of the ribozyme is tightly locked into a fixed conformation (this could be either a misfolded or a correctly folded conformation). In the lower path interdomain interactions form a “native trap” that stabilizes a misfolded catalytic core (11, 13). In an alternative (upper path) folding pathway (18), the extended form of P5abc capped by a GNRA tetraloop switches the open hand of L5c to a closed hand. This conformational change in L5c abolishes P14 and consequently facilitates the catalytic core rearrangement. Further folding may be required after the catalytic core becomes protected (9).