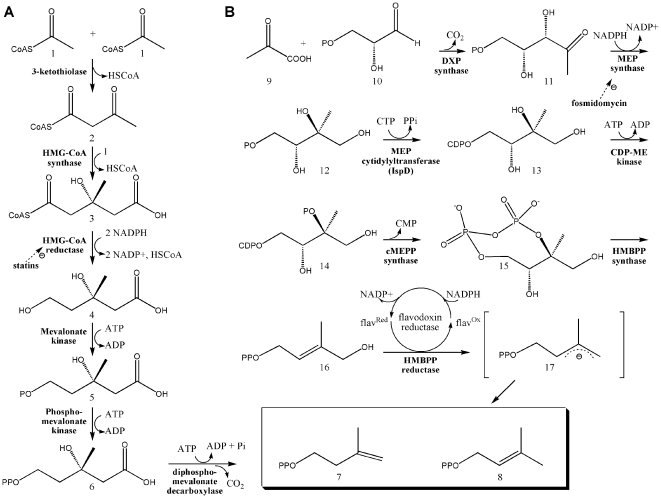
Figure 1. Two pathways of isoprene biosynthesis.
A) The MVA Pathway. Three molecules of acetyl-CoA (1) are condensed to form 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) (3), which is then reduced to MVA by HMG-CoA reductase (4) [20], [21]. MVA is further processed to yield IPP (7) [22]–[24], which is converted to DMAPP (8) by an isomerase [25]. B) The MEP Pathway. Pyruvate (9) is condensed with glyceraldehyde 3-phosphate (10) to yield 1-deoxy-D-xylulose 5-phosphate (DXP; (11)) [26], an intermediate with a role in E. coli vitamin B1 and B6 biosynthesis [27]–[30] as well as isoprene biosynthesis. 1-Deoxy-D-xylulose 5-phosphate reductoisomerase (also called MEP synthase) catalyzes the reduction and rearrangement of 11 to yield MEP (12) [31], the first committed step in the E. coli MEP pathway. The next enzyme, MEP cytidylyltransferase (or IspD), converts MEP into 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol (CDP-ME; (13)). CDP-ME kinase then phosphorylates CDP-ME, which becomes cyclized (coupled with the loss of CMP) by cMEPP synthase to yield 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (15) [14], [32]–[35]. A reductive ring opening of 15 produces 1-hydroxy-2-methyl-2-butenyl diphosphate (HMBPP; (16)) [36]–[40], which is reduced to both IPP and DMAP in a ∼5∶1 ratio [4], [41]–[46].