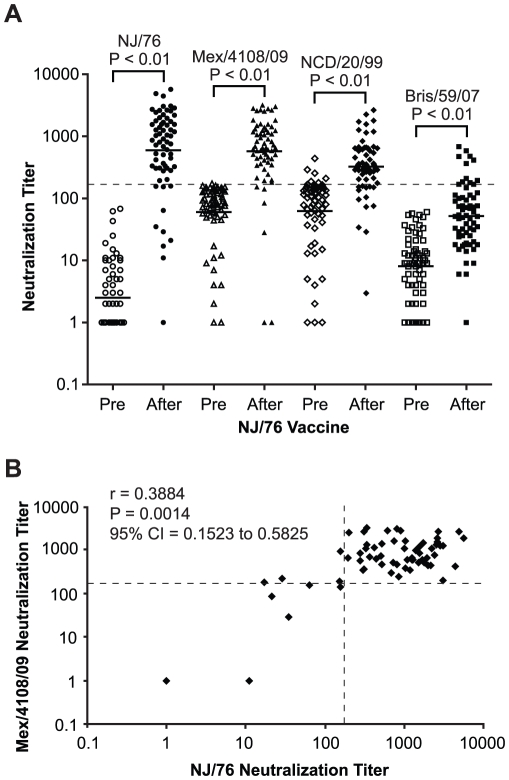
Figure 2. NJ/76 (swine flu) vaccination generates cross-neutralizing antibodies to 2009 H1N1 and seasonal influenza NCD/20/99.
(A) Neutralization titers to NJ/76, Mex/4108/09, NCD/20/99 and Bris/59/07 in NJ/76 clinical trial sera collected pre and post NJ/76 vaccination are shown. The geometric mean of titers (GMT) of neutralization in each group is indicated by the short line. P values were calculated by the comparison of pre and post (after) NJ/76 vaccination with paired t test. (B) Correlation between NJ/76 and Mex/4108/09 neutralizing titers in the sera of after NJ/76 vaccination was evaluated with the Spearman test for nonparametric correlation. r: Spearman r; P: two-tailed P value. The dotted lines in both panels A and B represent the neutralization titer of 160, which has been proposed as a correlate of seroprotection in microneutralization assays involving replicating influenza virus [4]. Protective titers based on neutralization of HA-pseudotypes have not been determined.