ABSTRACT
Tropical rainforests show the highest level of terrestrial biodiversity and may be an important contributor to microbial diversity. Exploitation of these ecosystems may foster the emergence of novel pathogens. We report the discovery of the first insect-associated nidovirus, tentatively named Cavally virus (CAVV). CAVV was found with a prevalence of 9.3% during a survey of mosquito-associated viruses along an anthropogenic disturbance gradient in Côte d’Ivoire. Analysis of habitat-specific virus diversity and ancestral state reconstruction demonstrated an origin of CAVV in a pristine rainforest with subsequent spread into agriculture and human settlements. Virus extension from the forest was associated with a decrease in virus diversity (P < 0.01) and an increase in virus prevalence (P < 0.00001). CAVV is an enveloped virus with large surface projections. The RNA genome comprises 20,108 nucleotides with seven major open reading frames (ORFs). ORF1a and -1b encode two large proteins that share essential features with phylogenetically higher representatives of the order Nidovirales, including the families Coronavirinae and Torovirinae, but also with families in a basal phylogenetic relationship, including the families Roniviridae and Arteriviridae. Genetic markers uniquely conserved in nidoviruses, such as an endoribonuclease- and helicase-associated zinc-binding domain, are conserved in CAVV. ORF2a and -2b are predicted to code for structural proteins S and N, respectively, while ORF3a and -3b encode proteins with membrane-spanning regions. CAVV produces three subgenomic mRNAs with 5′ leader sequences (of different lengths) derived from the 5′ end of the genome. This novel cluster of mosquito-associated nidoviruses is likely to represent a novel family within the order Nidovirales.
IMPORTANCE
Knowledge of microbial diversity in tropical rainforests is sparse, and factors driving the emergence of novel pathogens are poorly understood. We discovered and mapped the spread and genetic evolution of a novel mosquito nidovirus from a pristine rainforest to human settlements. Notably, virus diversity decreased and prevalence increased during the process of spreading into disturbed habitats. The novel virus, tentatively termed Cavally virus, contains genetic features common to members of the order Nidovirales (families Coronaviridae, Arteriviridae, and Roniviridae), including conservation of the replicase machinery and expression of subgenomic RNA messages, has a basal phylogenetic relationship to the family Coronaviridae, and clearly differs from the established nidovirus families. Inclusion of this putative novel family in the nidovirus phylogeny suggests that nidoviruses may have evolved from arthropods.
Introduction
Tropical rainforests comprise the highest level of terrestrial biodiversity (1–3). Microbes are dependent on their hosts, and hot spots of host biodiversity may also be rich in pathogen diversity. Intensive land use changes in West African rainforests began in the 1960s by industrial deforestation, leaving only remnants of the equatorial forest belt (4–6). Patches of forest are typically surrounded by agricultural land and human settlements. Logging is linked to a profound decline in biodiversity (4, 7–12). Declines in biodiversity are associated with an increased risk of infectious disease emergence in humans and domestic animals (reviewed in reference 13). Nevertheless, links between anthropogenic habitat modification and virus emergence remain to be confirmed (13, 14).
We recently conducted a survey of mosquito-borne viruses within and around a primary forest habitat in Côte d’Ivoire, West Africa (14). Findings of highly divergent viruses suggested an extension of the diversity of several virus taxa in primary forest habitats (15, 16). We also identified short sequence fragments of an unusual virus in insects that was distantly related to coronaviruses (14). Here we report the genomic sequence and organization of this first insect nidovirus and its evolutionary divergence along an anthropogenic disturbance gradient extending from primary forest into human settlements.
The order Nidovirales comprises the families Coronaviridae (subfamilies Coronavirinae [CoV] and Torovirinae [ToV]), as well as the monogeneric families Arteriviridae (ArV, genus Arterivirus) and Roniviridae (RoV, genus Okavirus) (17). Nidoviruses have a wide range of hosts, including crustaceans (RoV) (18), fish (ToV) (19), birds (CoV) (20), and a variety of mammals (ArV, CoV, and ToV) (17, 21–23). With plus-stranded genomes between 26 and 32 kb, CoV, ToV, and RoV have the largest known RNA genomes and are referred to as “large” nidoviruses (17). Genomes of the “small” nidoviruses (ArV) comprise 13 to 16 kb. All nidoviruses encode two replicase polyproteins, pp1a and pp1ab, encoded by open reading frame 1a (ORF1a) and ORF1b located at the 5′ end of the genome and followed by genes encoding structural proteins and, in most cases, several accessory (nonessential) proteins. A distinctive feature of nidoviruses is their transcription strategy. Genes downstream of the replicase polyprotein gene are expressed from a nested set of 3′-coterminal subgenomic mRNAs (in Latin, nidus means nest) (24–27). In this study, analyses of subgenomic mRNAs and major features of genome organization and phylogenetic analysis were employed for a taxonomic classification of a novel insect nidovirus. The summation of results suggests the discovery of a previously unrecognized family within the order Nidovirales.
RESULTS AND DISCUSSION
Prevalence and divergence of CAVV along a gradient of habitat modifications.
Between February and June 2004, 7,067 mosquitoes were trapped along an anthropogenic disturbance gradient in the area of the Taї National Park, Côte d’Ivoire. The gradient comprised sampling sites in the primary (pristine) forest, in secondary (modified) forest, in agriculturally exploited forest edge areas, and in adjacent human settlements (14–16). Initial analyses by cell culture and electron microscopy yielded viral particles with CoV-like morphology in supernatants of C6/36 cells infected with mosquito homogenates. A short genome fragment with low but significant identity to ORF1b of CoV was identified (14). This virus was tentatively named Cavally virus (CAVV), after a river near the sites where mosquitoes were caught.
Extensive attempts at virus isolation from adult female mosquitoes pooled in small numbers (up to 23 mosquitoes per pool) yielded virus in 40 (9.3%) of the 432 pools tested. As shown in Table 1, virus was most frequently isolated from Culex mosquitoes, especially Culex nebulosus. Mosquitoes of the genera Aedes, Anopheles, and Uranotaenia were also found to be infected but at lower rates. CAVV was present in all sampled habitat types but with the highest prevalence in human settlements (Table 1). Analysis of variance showed the observed virus isolation rate in human settlements (and only there) to be significantly different from the mean isolation rates (F test, P < 0.00001) and from all isolation rates in any other habitat. In order to gain insight into the genetic diversity of CAVV, a 603-nucleotide (nt) genome fragment representing the 1b polymerase gene region was amplified and sequenced from all positive pools. The pairwise genetic distance between the isolates was up to 15% at the nucleotide level and up to 9.9% at the amino acid level. In particular, the nucleotide distance of isolate CAVV/A4/CI/2004 from all other isolates ranged between 13.5 and 15%, whereas the range of distances was below 1.8% for all other CAVV isolates. This suggested the presence of a diversified virus population comprising two different clusters whose distance is compatible with different species.
TABLE 1 .
CAVV isolation from pools of female mosquitoes
| Sampling site | Pool | Mosquito species | No. of mosquitoes |
CPEa | % CAVV isolation (no. positive/total) |
Genetic distanceb (%) |
|---|---|---|---|---|---|---|
| Camps | A4 | Culex spp. | 20 | 3 dpi | 7.5 (5/67) | 0.1 |
| A21 | Culex (Eumelanomyia) spp. | 10 | Weak | |||
| A26 | C. nebulosus | 10 | 3 dpi | |||
| A28 | C. nebulosus | 22 | 7 dpi | |||
| A31 | Culex spp. | 9 | 2nd P, 7 dpi | |||
| Primary forest | B14 | Female, NDc | 17 | 1st P, 3 dpi | 4.1 (4/98) | 1.4 |
| B15 | C. nebulosus | 1 | 2nd P, 7 dpi | |||
| B40 | Female, ND | 2 | 3 dpi | |||
| B42 | C. nebulosus, C. cinerellus | 9 | 3 dpi | |||
| Secondary forest | C45 | C. nebulosus | 16 | 3 dpi | 5.1 (5/99) | 0.5 |
| C64 | Culex (Eumelanomyia) spp. | 10 | 7 dpi | |||
| C65 | Culex (Eumelanomyia) spp. | 8 | 3 dpi | |||
| C79 | Aedes harrisoni | 6 | 1st P, 7 dpi | |||
| C82 | Anopheles spp. | 20 | 2 dpi | |||
| Plantations | D27 | Aedes harrisoni | 2 | 7 dpi | 9 (9/100) | 0.1 |
| D28 | Anopheles spp. | 2 | 3 dpi | |||
| D42 | C. decens | 20 | 3 dpi | |||
| D50 | C. nebulosus | 20 | 3 dpi | |||
| D51 | C. nebulosus | 20 | 3 dpi | |||
| D60 | Female, ND | 15 | 1st P, 5 dpi | |||
| D61 | Female, ND | 11 | 1st P, 5 dpi | |||
| E9 | Uranotaenia chorleyi | 7 | 3 dpi | |||
| Human settlements | F2 | Female, ND | 23 | Weak | 26.5 (18/68) | 0.3 |
| F3 | Aedes harrisoni | 9 | 1st P, 5 dpi | |||
| F24 | C. nebulosus | 30 | 3 dpi | |||
| F25 | C. nebulosus | 21 | 3 dpi | |||
| F26 | C. nebulosus | 50 | 3 dpi | |||
| F27 | C. nebulosus | 40 | 1st P, 5 dpi | |||
| F28 | C. nebulosus | 20 | 3 dpi | |||
| F30 | C. nebulosus | 20 | 3 dpi | |||
| F32 | C. nebulosus | 20 | 3 dpi | |||
| F33 | C. nebulosus | 12 | 3 dpi | |||
| F35 | C. nebulosus | 16 | 3 dpi | |||
| F47 | Culicinae spp. | 10 | 8 dpi | |||
| F52 | C. quinquefasciatus | 20 | 3 dpi | |||
| F53 | C. quinquefasciatus | 8 | 1st P, 3 dpi | |||
| F54 | C. antennatus | 20 | 3 dpi | |||
| F55 | C. antennatus | 9 | 8 dpi | |||
| F56 | Culex spp. | 18 | 2nd P, 8 dpi | |||
| F63 | Anopheles spp. | 21 | 2nd P, 8 dpi |
Shown is the time point when the first CPE was visible. P, passage; dpi, days postinfection.
Maximal pairwise nucleotide distance with a 603-bp RT-PCR fragment in the ORF1b gene.
ND, not determined.
CAVV is an insect-associated virus whose hosts were encountered along a gradient of anthropogenic habitat modifications. To examine possible influences of habitat modification on virus diversity, average nucleotide distances over sequence pairs were determined within the amplified 603-nt fragments from all pools and were assigned to one of the habitat types from where the respective isolates originated, i.e., (i) primary forest or research camps within a primary forest (9 isolates), (ii) secondary forest (5 isolates), (iii) plantations (6 isolates), and (iv) villages (19 isolates). The mean numbers of nucleotide exchanges in pairwise sequence comparisons were determined within the samples pertaining to each habitat type. CAVV/A4/CI/2004 was excluded to prevent bias from a virus that may be evolutionarily disconnected from the main clade (putative second species). In habitat types i to iv, the mean pairwise exchange rates within the 603-nt fragment were 4.35, 2.8, 0.6, and 2, respectively. Even though isolates from villages predominated numerically in the data set, the level of diversification was highest in primary forest (chi-square test on exchange rates, P < 0.01).
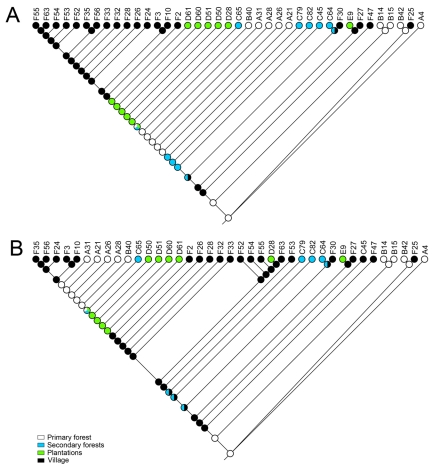
To estimate the habitat association of the putative common ancestor of the 39 virus isolates, sequence fragments were subjected to phylogenetic analysis using a minimum evolution model, considering either the numbers of pairwise synonymous nucleotide exchanges or the percentages of overall (synonymous and nonsynonymous) pairwise nucleotide exchanges. CAVV/A4/CI/2004 was used as an outgroup. Phylogenies were subjected to ancestral state reconstruction under a parsimony assumption. These simple models of phylogeny and trait evolution were chosen because the ordered and sparse pattern of nucleotide exchange across the alignment suggested the absence of multiple or saturating exchanges. As shown in Fig. 1, ancestral state reconstruction suggested the most recent common ancestor linked to the outgroup, as well as the common ancestor of the ingroup, to have existed in a primary forest habitat. This matches the general concept wherein novel viruses are being transferred to areas of human settlement in the course of agricultural exploitation of primary habitats.
FIG 1 .
CAVV ancestral state reconstruction based on a maximum-parsimony algorithm. Minimum evolution phylogenies based on the numbers of synonymous exchanges (A) and the percent nucleotide distance (B) were calculated in Mega 5 (66). CAVV strain A4 served as an outgroup. Tip traits in Mesquite (http://mesquiteproject.org/mesquite/mesquite.html) were defined by the habitats from which the virus isolates were taken (identified by color code at the bottom left of panel B). The circles at each root point identify by colored segments the likelihood of the root point taxon having existed in the habitat shown.
We tested 269 pools of 1,716 adult male mosquitoes collected during this survey for CAVV infection by specific real-time reverse transcription (RT)-PCR (14). CAVV was not detected in male mosquitoes, suggesting that CAVV is likely dependent on amplifying vertebrate hosts.
Viral growth and morphology.
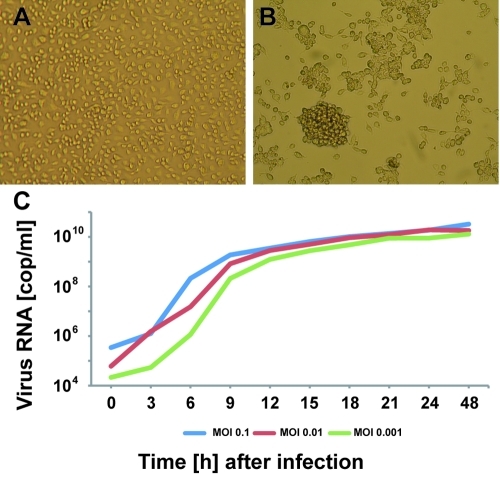
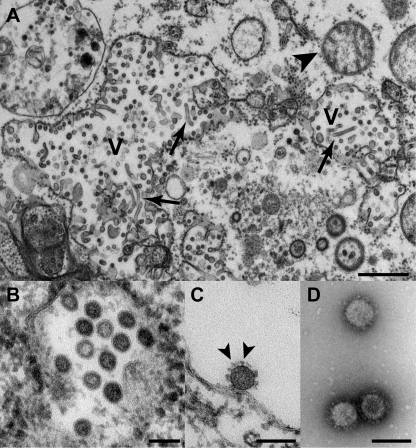
Virus growth kinetics and morphological presentation in insect cells were studied. Cells showed strong cytopathic effects (CPE) manifesting in aggregation of cells at 48 h postinfection (hpi) (Fig. 2A and B). Virus replication was measured by real-time RT-PCR every 3 h for 2 days. Maximal RNA concentrations were reached at 15 to 18 hpi, indicating a fast replication cycle (Fig. 2C). Enveloped, spherical CoV-like virions with a mean diameter of 120 nm and large, club-shaped surface projections were detected in cell culture supernatants at 48 hpi by negative staining electron microscopy (Fig. 3). In ultrathin sections of fixed insect cells analyzed by transmission electron microscopy at 48 hpi, vesicles containing spherical, potentially enveloped particles were observed in the cytoplasm of infected cells. These were 50 to 60 nm in diameter and lacked surface projections (Fig. 3A and B). A role for these vesicles filled with virions resembling steps during virus maturation, as shown for other plus-strand RNA viruses, remains to be investigated (28–30). Furthermore, tubular structures likely of viral origin were detected in the cytoplasm of infected cells (Fig. 3A). Separation or adsorption of putative virions on cell membranes is shown in Fig. 3C. These particles were morphologically indistinguishable from typical particles encountered in cleared cell culture supernatant (Fig. 3D).
FIG 2 .
CAVV growth on insect cells. CPE in C6/36 cells infected with CAVV was observed at 48 hpi (B) compared to mock-infected C6/36 cells (A). Numbers of CAVV genome copies per milliliter in cell culture supernatant of C6/36 cells infected with CAVV at MOIs of 0.1, 0.01, and 0.001 were measured by RT-PCR at 0 to 48 hpi (C).
FIG 3 .
CAVV replication and morphology as observed by transmission electron microscopy. Ultrathin sections of C6/36 cells infected with CAVV at 48 hpi (A to C), showing an overview of the cytoplasm of infected cells (A; bar, 1 µm; V, vesicle with virus formation; arrowhead, mitochondrion; arrow, tubular structures likely of viral origin) and a higher magnification of vesicles containing spherical, potentially enveloped particles (B; bar, 100 nm) and separation or adsorption of putative virions on cell membranes (C; bar, 100 nm; arrowhead, spikes on virus surface). Negative staining (1% uranyl acetate) of CAVV sedimented by ultracentrifugation through a 36% sucrose cushion (D; bar, 100 nm). It should be noted that better EM results were obtained at 48 hpi than at 24 hpi.
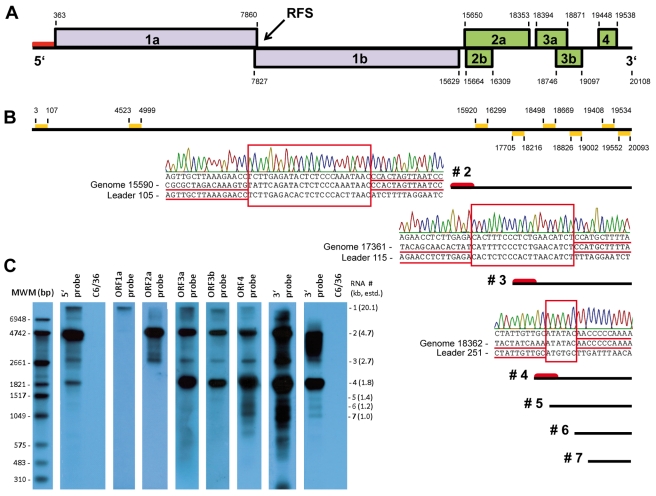
Full genome sequencing of isolate CAVV/C79/CI/2004 was achieved by a combination of adaptor-based random RT-PCR and ultradeep sequencing. The entire CAVV genome comprised 20,108 nt, excluding the 3′ poly(A) tail (GenBank accession number HM746600), a size intermediate between that of arteriviruses (13 to 16 kb) and CoV or RoV (26 to 32 kb) (17). The genome contained seven major ORFs, as well as untranslated regions of 362 and 570 nt at the 5′ and 3′ ends, respectively (Table 2; Fig. 4A). In an effort to identify potential functional domains, the seven ORFs were compared by psiBLAST to a database restricted to nidoviruses (Table 2).
TABLE 2 .
Predicted genome organization of CAVV
| ORF | Frame | Genome position (nt) |
No. of amino acids |
Predicted protein size (kDa) |
Predicted protein |
|---|---|---|---|---|---|
| 1a | +3 | 363–7862 | 2,500 | 288 | Polyprotein 1a |
| 1b | +2 | 7826–15628 | 2,600 | 301 | ORF1b-encoded part of polyprotein 1ab |
| 2a | +2 | 15650–18352 | 900 | 103 | Spike protein |
| 2b | +1 | 15664–16308 | 214 | 24 | Nucleocapsid protein |
| 3a | +1 | 18394–18870 | 158 | 18 | Membrane protein |
| 3b | +2 | 18746–19096 | 116 | 14 | Membrane protein |
| 4 | +2 | 19399–19548 | 50 | 6 | Unknown |
FIG 4 .
CAVV genome organization and subgenomic mRNA synthesis. (A) Positions and sizes of CAVV ORFs. (B) Positions of probes used for Northern blotting and placement of putative sgRNAs. Putative leader sequences are marked by red bars. Electropherograms shown next to mRNAs 2, 3, and 4 indicate typical leader-body fusion sites identified by RT-PCR. No clear leader-body fusions were identified for RNAs shown without leader symbols (see Fig. S2 in the supplemental material). (C) Detection of CAVV genome and sgRNAs by Northern blot analysis of intracellular viral RNA from infected C6/36 cells. Specific probes for the 5′ and 3′ prime ends, as well as for each ORF, were employed. The 3′-terminal probe is shown after short and long exposures of the blot. A molecular size marker (MWM) is shown in the left lane. Molecular size indicators on the right summarize estimated sizes of putative subgenomic mRNAs.
Putative replicase polyprotein genes.
The replicase genes of nidoviruses share several domains and features conserved across the families Arteriviridae, Coronaviridae, and Roniviridae (25). These are (from the N to the C terminus) transmembrane domain 1 (TMD1), TMD2, a 3C-like protease (3CLpro) (31), TMD3, a ribosomal frameshift site (RFS) (32, 33), an RNA-dependent RNA polymerase (RdRp) (34), a zinc-binding domain (ZBD), an RNA helicase (HEL), and a uridylate-specific endoribonuclease (NendoU) (35, 36). CoV, ToV, and RoV also share a 3′-5′ exonuclease (ExoN) upstream of NendoU (37) and a ribose-2′-O-methyltransferase (MT) at the C terminus (38, 39). CoV and ToV also encode an ADP-ribose 1-phosphatase upstream of TMD1 (19, 40, 41).
Using TMHMM v2.0, three hydrophobic regions comprising putative multiple membrane-spanning domains (TMD1 25L-L47, TMD2 1128I-Y1272, and TMD3 1727Y-M1780) were identified within the first predicted ORF of the CAVV genome. Three TMDs are also found in ORF1a of CoV and ToV, whereas RoV has four TMDs (18). However, the position of CAVV TMD1 was similar to that in RoV (18). Between TMD2 and TMD3, a putative 3CLpro domain was identified. Comparative sequence analysis suggested that the CAVV 3CLpro domain is a cysteine protease with a Cys-His-Asp catalytic triad (see Fig. S1a in the supplemental material).
Putative functional motifs with similarity to CoV and ToV in the second ORF included an RdRp domain (631Y-V787, cd01699), a ZBD (1051C-L1113, UPF1, pfam09416, Fig. S1b), and a HEL domain (1466K-I1717, superfamily I DNA and RNA helicases, COG1112). Phylogenies of RdRp and HEL domains indicated equidistant basal relationships to CoV and RoV (see below). Furthermore, a putative NendoU was identified. Sequence alignments suggest that the active site of the CAVV NendoU involves residues His4670, His4685, and Lys4725 (Fig. S1c). Although short regions with low similarity suggest conservation of the ExoN and MT domains, reliable alignments could not be generated and biochemical evidence remains to be obtained to confirm the functionality of these domains.
Putative RFS.
Expression of the second major ORF of nidoviruses involves a programmed ribosomal frameshift into the −1 reading frame, occurring just upstream of the ORF1a stop codon (32, 33). The overlap region of ORF1a and -1b typically contains a slippery heptanucleotide sequence and a downstream RNA pseudoknot structure that together promote ribosomal frameshifting (32, 33). With minor variations, previously identified nidovirus slippery sequences conform to the XXXYYYZ consensus sequence conserved in many ribosomal slip sites on viral RNAs (for a review, see reference 42). Thus, for example, ArV, CoV, and ToV use 5′-(U/G)UUAAAC and RoV uses 5′-AAAUUUU as a slippery sequence (18, 42). The short (~35-nt) ORF1a/1b overlap region in CAVV does not contain heptanucleotide sequences related to those of other nidoviruses. The only XXXYYYZ-like sequence identified in this region of the CAVV genome is 7829CCCUUUG. However, previous systematic mutagenesis studies of ribosomal slip sites (43) revealed that the CCCUUUG heptanucleotide sequence does not promote efficient −1 ribosomal frameshifting in vitro and, to our knowledge, has not been reported to mediate efficient ribosomal frameshifting in viral or cellular systems. Further analyses revealed 7835GGAUUUU as a further candidate slip site. The GGAUUUU sequence conforms to the simultaneous-slippage model introduced by Jacks et al. (44). More importantly, data obtained for red clover necrotic mosaic (diantho) virus (RCNMV) (45, 46) have shown that this sequence mediates efficient −1 ribosomal frameshifting and thus expression of the downstream p57 polymerase ORF of RCNMV. Furthermore, the CAVV 7835GGAUUUU sequence is located 5 nt upstream of an energetically favorable RNA secondary structure. Both the length of the spacer element and the presence of a putative stem-loop structure adjacent to the proposed frameshift site support the idea that CAVV ORF1b expression is mediated by −1 ribosomal frameshifting at this site. Even though direct experimental evidence would be desirable to further corroborate this prediction, it seems reasonable to suggest that ORF1a/1b-encoded sequences are fused at a 2491Leu-Asp-Phe-Ser junction site.
Regulation of transcription.
Although all nidovirus subgenomic mRNAs are 3′ coterminal, different families and genera have different subgenomic mRNA 5′ ends. ArV and CoV subgenomic mRNAs contain a common 5′ leader sequence derived from the 5′ terminus of the genomic RNA. The leader template is fused to nascent minus strand mRNA templates via a copy choice-related template switching process called discontinuous extension of minus strands or discontinuous transcription (DT) (47, 48). Copy choice occurs at transcription-regulating sequences (TRS), comprising short conserved sequence motifs that follow the leader and precede each downstream gene’s ORF (48–50). In contrast, the subgenomic mRNAs of RoV do not contain leader sequences (24). Synthesis of subgenomic mRNA templates is believed to be mediated solely by attenuation of minus strand synthesis, involving mRNA transcription on minus-stranded replicative intermediates (nondiscontinuous transcription [NDT]) (26). ToV uses DT to express RNA2 and NDT for RNAs 3 through 5, respectively (51).
To investigate the nature of subgenomic mRNAs in CAVV, total RNA was isolated from CAVV-infected cells and subjected to Northern blot analysis. RNA from noninfected C6/36 cells served as a control. To avoid detection of defective interfering RNAs, cells were infected at a low multiplicity of infection (MOI) with virus obtained from limiting dilution endpoints of early-passage supernatants. Northern blot probes were generated against the most 5′ 107 nt and the most 3′ 556 nt of the genome (Fig. 4B). Additional probes were generated for the major predicted ORFs (Fig. 4B). Apart from a band corresponding to the genome size, fragments of approximately 4.7, 2.7, and 1.8 kb were detected with the 5′ probe. These were also represented in a blot with the 3′ probe, suggesting that these RNAs are 5′ and 3′ coterminal with the genome (DT, Fig. 4C). Additional bands of ca. 1.4, 1.2, and 1.0 kb were detected with the 3′ probe but not with the 5′ probe, compatible with an NDT mechanism.
The ORF2a probe detected the genome and two additional bands, one corresponding to a subgenomic RNA (sgRNA) starting upstream of ORF2a, the other within ORF2a (Fig. 4A). No band corresponding to a separate sgRNA for the predicted ORF2b was observed. A prominent band of 1.8 kb was seen with all probes in and downstream of ORF3a, suggesting an sgRNA starting upstream of ORF3a. A minor band of 1.4 kb was confirmed with the probe placed in ORF3b and all downstream probes. Two additional minor bands of ca. 1.2 and 1.0 kb were seen with the ORF4 probe and the 3′-end probe. According to estimated molecular weights, these corresponded to sgRNAs starting ca. 300 and 500 nt upstream of the initiation codon of ORF4 (19399AUG). Functional studies are required to determine these positions more precisely, as size estimates are based on RNA gel electrophoresis only.
To identify potential fusion sites indicative of DT, one-step RT-PCRs were conducted for each sgRNA detected by Northern blotting. Sense primers were placed at intervals starting from the 5′ end of the genome approximately 300 nt into the genomic sequence (see Fig. S2 in the supplemental material). Antisense primers were selected according to the projected sizes of the sgRNAs. RT-PCRs targeting the 4.7-kb and 2.7-kb bands resulted in products with sense primers up to at least 126 and 152 nt, respectively, from the 5′ end of the genome. The 1.8-kb band was associated with PCR products up to 202 nt from the 5′ end of the genome. PCR products of various and unexpected sizes were observed for the smaller potential sgRNAs (i.e., all sgRNAs smaller than the 1.8-kb band in the Northern blot). Representative PCR products for all putative sgRNAs were cloned and sequenced. PCR products of unexpected sizes from small subgenomic mRNAs yielded sequences indicative of misprimed amplification in various positions of the genome, and it was concluded that these subgenomic mRNAs were generated by NDT, lacking a leader consistent with the Northern blotting results.
The three sgRNAs that were clearly codetected with 5′ and 3′ probes revealed potential sites of fusions between genome leader and downstream mRNA sequences. Different sites were used for each sgRNA, as shown in Fig. 4B. Nevertheless, all sgRNAs had an A/C-rich region immediately downstream of the fusion region in common. It should be noted that Northern blot detection intensities of the 4.7-kb and 2.7-kb bands corresponded well between the 5′ and 3′ probes, whereas the detection intensity of the 1.8-kb band was much greater with the 3′ probe than with the 5′ probe (Fig. 4C). This matched the presence of a much smaller fusion region in the RT-PCR product of the 1.8-kb sgRNA. Given the high intensity of the 1.8-kb band, we suspected that a major fraction of the total amount of this sgRNA would not contain a fused leader element; nonetheless, several variant fusion sites were detected in parallel clones for all subgenomic mRNAs. Further studies are required to characterize in more detail the various CAVV RNA species and their functional relevance in CAVV genome expression.
Initial predictions on structural protein genes.
Sequence analyses of proteins predicted to be expressed from the 5 major ORFs in the 3′-proximal region of the CAVV genome revealed little (if any) similarity with other viral (and cellular) proteins, confirming that CAVV diverged profoundly from other nidoviruses and complicating functional assignments of these proteins. As summarized in Table 2, two proteins are expected to be expressed from sgRNA 2. ORF2a is predicted to encode a type I glycoprotein featuring a C-terminal membrane-spanning domain and multiple glycosylation sites. Based on these predictions, the protein likely represents a functional equivalent of the S protein of other nidoviruses, which remains to be confirmed in further studies. The predicted translation start codon of ORF2b is the second AUG on the subgenomic mRNA2, located just downstream of the ORF2a start codon, suggesting that ORF2b may be translated by a leaky scanning mechanism. ORF2b encodes a highly basic protein (pKa, 10.8) with a molecular mass of 24 kDa. Both its size and its charge suggest that this protein may be the viral nucleocapsid protein. Among nidoviruses, this upstream position of the (presumed) N protein gene in the CAVV genome is unusual but has its precedent in members of the family Roniviridae (52). ORF3a and -3b encode proteins with predicted molecular masses of 18 and 14 kDa, respectively. Database searches failed to reveal close homologs of these proteins. Protein analysis software predicts the presence of membrane-spanning domains in both proteins (residues 95 to 117 in ORF3a and residues 73 to 95 in ORF3b), suggesting that both proteins are integral membrane proteins. The ORF3a protein likely contains a signal peptidase cleavage site, 15Ala-Met-Ser|Ala-Glu, and is predicted to be glycosylated, further supporting a role as a membrane-spanning structural protein of the virus. The specific mechanism used to express the ORF3b gene product is unclear but may involve internal ribosomal entry, as shown previously for several downstream ORFs expressed from coronavirus subgenomic mRNAs (53–56). Further studies are required to establish if ORF3a and ORF3b proteins have functions related to those of the membrane-spanning M and E proteins of other nidoviruses. ORF4 encodes a small protein of 50 amino acid residues with unknown functions.
Phylogenetic analyses reveal a novel cluster of insect nidoviruses.
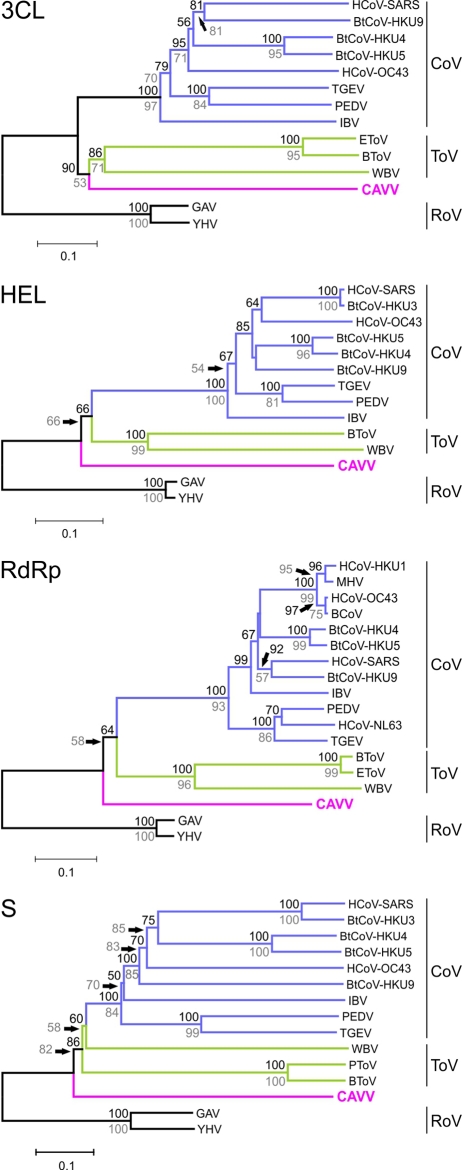
In order to identify the phylogenetic relationship of CAVV with other nidoviruses, we conducted phylogenetic analyses of protein alignments of conserved motifs within 3CLpro (ORF1a), RdRp, and HEL (ORF1b), as well as the putative structural S protein (Fig. 5). In all phylogenies, CAVV branched from a deep node in the nidovirus tree above the Roniviridae family. In the ORF1b and S genes, CAVV had a most recent ancestor in common with the subfamilies Torovirinae and Coronavirinae. In 3CLpro, it branched from an ancestor it has in common with the subfamily Torovirinae. The low bootstrap support at this root point suggested that CAVV might also branch from an ancestor it has in common with Torovirinae and Coronavirinae. Although formal classification criteria for nidoviruses are not established, this phylogenetic positioning suggested CAVV to be a profoundly separated cluster of nidoviruses that might constitute a new family of nidoviruses with a phylogenetic position between Roniviridae and Coronaviridae on one side and Arteriviridae on the other.
FIG 5 .
Phylogenetic relationship of CAVV to prototype nidovirus strains for selected genomic regions. Phylogenetic analyses were performed using the NJ algorithm, a BLOSUM62 substitution matrix, and no distance correction. Indels were fully deleted. Significance was tested by bootstrap analysis using 1,000 resampling steps as implemented in MEGA 5.0 (66). Bootstrap values are shown above nodes. Analyses were also performed using the maximum-likelihood algorithm in FastML with the same settings. Results of this analysis are not shown because of congruent topologies. Bootstrap support values (1,000 replicates) from maximum-likelihood analysis are shown in grey below nodes.
Conclusions
CAVV is the first mosquito nidovirus and represents the prototype species of a family in the order Nidovirales that includes features distinct from those established for the Arteriviridae, Roniviridae, and Coronaviridae. Based on morphology, conserved genome motifs, and phylogenetic relationship, CAVV cannot be assigned to one of the established nidovirus families. Further investigations are required to elucidate further details of the CAVV replication apparatus and structural protein functions. It is unknown whether CAVV infection is restricted to mosquitoes or if transmission to other hosts, potentially vertebrates, occurs. It is interesting that CoV has not been detected in insects, but that the typical reservoir hosts—bats for alpha- and beta-CoV and birds for gamma-CoV—are largely insect feeding. Common ancestors of CAVV and CoV may thus have been insect borne and have diverged after independent host switches to bats and birds. This is in contrast to earlier proposals suggesting acquisition of gamma-CoV by birds from bats via raptors (57) but in agreement with hypotheses that emphasize CoV phylogeny and ecological considerations (58). Moreover, it has been suspected from epidemiological observations that a link between ToV and insects may exist (59). Even though it has not been confirmed that these viruses are carried by insects, the epidemiological implications of insects suggest at least ecologically relevant contact between the virus and its host. An ancestral existence of nidoviruses in arthropods is also supported by the phylogeny of the Nidovirales CAVV included; phylogenetically basal RoV is hosted by crustaceans, which, like mosquitoes, belong to the phylum Arthropoda. An arthropod host at the root of the Nidovirales tree would provide a parsimonious explanation for host associations of several of the known Nidovirales.
Our data on CAVV prevalence and divergence demonstrate how a virus may evolve through emergence from a pristine rainforest habitat into surrounding areas of less host biodiversity due to anthropogenic modification. Critically, while extending out of the primary forest habitat, the virus seemed to narrow its genetic diversity while increasing in prevalence (reverse dilution effect [13, 60, 61]). Further investigations are needed to untangle if the higher prevalence of CAVV in human settlements relates to a higher density of hosts (insects or vertebrates) or virus adaption. This principal understanding is necessary to develop experimental ecology models of virus-vector dynamics. This study underlines the importance of linking ecosystem biology and virus ecology to unravel the role of ecosystem modifications in the emergence of novel pathogens.
MATERIALS AND METHODS
Virus isolation and purification.
Virus isolation from 432 pools of 4,839 female mosquito heads was done with Aedes albopictus (C6/36) cells as described previously (15). For virus growth kinetics, C6/36 cells were infected with an MOI of 0.1, 0.01, or 0.001 and incubated for 1 h at 28°C. The inoculum was removed, and cells were washed with phosphate-buffered saline (PBS). L-15 medium was added, and cells were incubated for 48 h. Every 3 h, an aliquot of the cell culture supernatant was removed, RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), cDNA was synthesized using the SuperScript III RT System (Invitrogen, Karlsruhe, Germany), and CAVV viral genome copy numbers were quantified by real-time RT-PCR (14).
To obtain pure virus stocks, CAVV was titrated on insect cells and cell culture supernatant was harvested at 22 hpi to allow only single-round infections. Numbers of genome copies were measured by real-time RT-PCR, and the infectious supernatant of the highest dilution still showing virus replication was used for retitration of CAVV on insect cells. This procedure was repeated five times.
For purification, CAVV was harvested by freeze-thawing of infected cells. Cell debris was removed by centrifugation at 3,000 rpm for 20 min, followed by ultracentrifugation through a 36% sucrose cushion at 35,000 rpm (SW40 rotor; Beckman) for 2 h at 4°C. The virus pellet was suspended in 150 µl PBS overnight at 4°C. Further purification was achieved on a continuous gradient of 1 to 2 M sucrose in 0.01 M Tris-HCl–4 mM Na-EDTA at 35,000 rpm (SW40 rotor; Beckman) for 22 h at 4°C. Virus-containing fractions were tested by real-time RT-PCR, and fractions with the highest sequence titers were concentrated through a 36% sucrose cushion at 35,000 rpm (SW40 rotor; Beckman) for 2 h at 4°C. The virus pellet was suspended in 150 µl PBS buffer overnight at 4°C.
Electron microscopy.
For electron microscopy, viral particles were purified through a cushion of 36% sucrose and the pellet was suspended in PBS (15, 16). Viral particles were fixed with 2% paraformaldehyde and analyzed by transmission electron microscopy after negative staining with 1% uranyl acetate (62, 63). For ultrathin sections, infected cells were fixed with 2.5% glutaraldehyde, enclosed in low-melting-point agar, embedded in resin, and evaluated by transmission electron microscopy after ultrathin sectioning.
Genome sequencing.
RNA extracted from purified virus preparations was used for unbiased high-throughput sequencing and for conventional sequencing approaches. Genome fragments were generated by adaptor-based random RT-PCR. Following protocols described previously (15, 64), in this specific study, random hexamers linked to a defined primer sequence tail or oligonucleotides that bind to the conserved TRS elements of CoV linked to an oligonucleotide anchor were applied (see Table S1 in the supplemental material).
Genome characterization and phylogenetic analyses.
The nucleotide sequence of the CAVV genome was analyzed for ORFs and translated. Nucleotide and amino acid sequences were compared with other sequences by BLASTn, BLASTx, tBLASTx, and psiBLAST with the GenBank database (http://www.ncbi.nlm.Nih.gov/Genbank), and protein motifs were identified by web-based comparison to the Pfam database (http://www.pfam.janelia.org). Identification of cleavage sites for signal peptides was accomplished by using signalP-NN (http://www.cbs.dtu.dk/services/SignalP). Prediction of the hydropathy profile was performed by TMHMM v2.0 (http://www.cbs.dtu.dk/services/TMHMM/), and N-linked glycosylation sites were identified using the NetNGlyc 1.0 server (http://www.cbs.dtu.dk/services/NetNGlyc). RNA folding was modeled by using the Mfold server (http://mfold.bioinfo.rpi.edu/cgi-bin/rna-form1.cgi) (65). For phylogenetic analysis, CAVV amino acid sequences were aligned with representative sequences of other nidoviruses in MEGA v5.0 (66). Alignments were optimized according to published crystal structure predictions. Phylogenetic analysis of amino acid sequences was conducted by the neighbor-joining (NJ) algorithm with the BLOSUM62 substitution matrix for distance correction with 1,000 bootstrap replicates in MEGA v5.0. Maximum-likelihood analyses were done with Fasttree (67), and tree files were displayed in MEGA versus 5.0. Evolutionary divergence over sequence pairs was estimated in MEGA versus 5.0.
Identification of subgenomic mRNAs.
For Northern blotting, the Northern Blot Starter Kit (Roche, Mannheim, Germany) was used. Digoxigenin (DIG)-labeled probes were generated by PCR using the primers shown in Fig. 4B and listed in Table S1 in the supplemental material. Total RNA of isolate CAVV/C79/CI/2004 was extracted with the Qiagen RNeasy Kit (Qiagen, Hilden, Germany) from C6/36 cells at 24 hpi. RNA was separated on a 2% formaldehyde–1.5% agarose gel, blotted onto a nylon membrane (Roche, Mannheim, Germany), and hybridized with the CAVV-specific, DIG-labeled probes. RNAs were analyzed by chemiluminescence using 1:10,000 anti-DIG–alkaline phosphatase Fab fragments and 1:100 CDP-Star reagent (Roche, Mannheim, Germany).
SUPPLEMENTAL MATERIAL
Multiple sequence alignments of putative CAVV replicative proteins. (A) Alignment of astro-, poty-, and nidovirus 3C-like protease domains. Putative catalytic Cys, His, and Asp/Glu residues are indicated by asterisks. Also indicated are conserved residues predicted to be part of the S1 specificity subsite (&). (B) Alignment of CAVV and coronavirus helicase-associated N-terminal ZBDs. Putative zinc-binding residues are indicated (#). (C) Alignment of nidoviral endoribonuclease (NendoU) domains. Putative catalytic residues (His, His, Lys) are indicated by asterisks. Partial pp1a/pp1ab sequences were aligned using ClustalX 2.0 (Larkin MA, et al., Bioinformatics 23:2947–2948, 2007) and rendered with ESPript 2.2 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi) (Gouet P, Courcelle E, Stuart DI, Metoz F, Bioinformatics 15:305–308, 1999). Abbreviations of virus names and accession numbers are as follows: BToV, bovine torovirus (strain Breda 1) (AY427798); EToV, equine torovirus (strain Berne) (X52374); WBV, white bream virus (strain DF24/00) (NC_008516); EAV, equine arteritis virus (strain Bucyrus) (NP_127506); PRRSV, porcine reproductive and respiratory syndrome virus (strain Lelystad) (Q04561); LDV, lactate dehydrogenase-elevating virus (neurovirulent type C) (Q06502); GAV, gill-associated virus (AF227196); TEV, tobacco etch virus (AAA47909); HAstV-1, human astrovirus 1 (AAW51880); HCoV-229E, human coronavirus 229E (NC_002645); HCoV-NL63, human coronavirus NL63 (NC_005831); TGEV, transmissible gastroenteritis virus (strain Purdue-115) (Z34093); MHV-A59, murine hepatitis virus (strain A59) (NC_001846); SARS-CoV, severe acute respiratory syndrome-related CoV (strain Frankfurt-1) (AY291315); HCoV-OC43, human CoV strain OC43 (AY585229); IBV, avian infectious peritonitis virus (strain Beaudette) (NC_001451). Download Figure S1, PDF file, 0.420 MB.
CAVV leader-to-body fusion sites. Leader-to-body fusion sites of CAVV subgenomic mRNAs were identified using the SuperScript III One-Step RT-PCR System with Platinum Taq High Fidelity (Invitrogen, Karlsruhe, Germany) with leader-specific primers in combination with subgenomic-mRNA-specific reverse primers (primers are indicated by arrowheads) (see Table S1). Primers that yielded no PCR products are shown in grey. PCR products were sequenced and, in the case of unclear sequencing results, TA cloned into the pCR2.1 TOPO vector (Invitrogen, Karlsruhe, Germany) and resequenced. Download Figure S2, JPG file, 0.315 MB.
CAVV primers used in this study.
ACKNOWLEDGMENTS
We thank G. Gagné for field assistance and the Ivorian authorities for long-term support, especially the Ministry of the Environment and Forest, as well as the Ministry of Research, the directorship of the Taї National Park, and the Swiss Research Center in Abidjan. We are grateful for logistic support by the Taї chimpanzee project and C. Boesch. We thank A. Kopp, M. Marklewitz, A. Tashmukhamedova, and A. Petrosov for technical assistance.
This project was supported by the Robert Koch Institute and the European Union DG Research through the programs EMPERIE (grant agreement 223498) and EVA (grant agreement 228292), by the Deutsche Forschungsgemeinschaft (grant agreement DR772/3-1), and by the BonFor program of the University of Bonn (grant agreement O-156-0006). Work at the Center for Infection and Immunity at Columbia University was supported by the National Institutes of Health (AI079231, AI079231), the United States Department of Defense, and the United States Agency for International Development (USAID) Emerging Pandemic Threats (EPT) Program, PREDICT project, under the terms of cooperative agreement GHN-A-OO-09-00010-00.
Footnotes
Citation Zirkel F, et al. 2011. An insect nidovirus emerging from a primary tropical rainforest. mBio 2(3):e00077-11. doi:10.1128/mBio.00077-11.
REFERENCES
- 1. Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Tai forest: behavioural ecology and evolution. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 2. Myers N. 1990. The biodiversity challenge: expanded hot-spots analysis. Environmentalist 10:243–256 [DOI] [PubMed] [Google Scholar]
- 3. Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853–858 [DOI] [PubMed] [Google Scholar]
- 4. Chapman CA, Balcomb SR, Gillespie TR, Skorupa JP, Struhsaker TT. 2000. Long-term effects of logging on African primate communities: a 28-year comparison from Kibale National Park, Uganda. Conserv. Biol. 14:207–217 [Google Scholar]
- 5. Hall JS, Harris DJ, Medjibe V, Ashton PMS. 2003. The effects of selective logging on forest structure and tree species composition in a Central African forest: implications for management of conservation areas. Forest Ecol. Manag. 183:249–264 [Google Scholar]
- 6. Van Gemerden BS, Olff H, Parren MPE, Bongers F. 2003. The pristine rain forest? Remnants of historical human impacts on current tree species composition and diversity. J. Biogeogr. 30:1381–1390 [Google Scholar]
- 7. Doherty PF, Grubb TC. 2002. Survivorship of permanent-resident birds in a fragmented forested landscape. Ecology 83:844–857 [Google Scholar]
- 8. Gascon C, Williamson GB, da Fonseca GA. 2000. Ecology. Receding forest edges and vanishing reserves. Science 288:1356–1358 [DOI] [PubMed] [Google Scholar]
- 9. Meyer CFJ, Fründ J, Lizano WP, Kalko EKV. 2008. Ecological correlates of vulnerability to fragmentation in neotropical bats. J. Appl. Ecol. 45:381–391 [Google Scholar]
- 10. Morgan K, et al. 2009. Molecular phylogenetics and biogeography of the Neocellia Series of Anopheles mosquitoes in the Oriental Region. Mol. Phylogenet. Evol. 52:588–601 [DOI] [PubMed] [Google Scholar]
- 11. Thomas CD. 2000. Dispersal and extinction in fragmented landscapes. Proc. Biol. Sci. 267:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wade TG, Riitters KH, Wickham JD, Jones KB. 2003. Distribution and causes of global forest fragmentation. Conserv. Ecol. 7:7 [Google Scholar]
- 13. Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Junglen S, et al. 2009. Examining landscape factors influencing relative distribution of mosquito genera and frequency of virus infection. Ecohealth 6:239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Junglen S, et al. 2009. A new flavivirus and a new vector: characterization of a novel flavivirus isolated from Uranotaenia mosquitoes from a tropical rain forest. J. Virol. 83:4462–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quan PL, et al. 2010. Moussa virus: a new member of the Rhabdoviridae family isolated from Culex decens mosquitoes in Cote d’Ivoire. Virus Res. 147:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siddell SG, Ziebuhr J, Snijder EJ. 2005. Coronaviruses, toroviruses and arteriviruses, 10th ed Hodder Arnold, London, United Kingdom [Google Scholar]
- 18. Cowley JA, Dimmock CM, Spann KM, Walker PJ. 2000. Gill-associated virus of Penaeus monodon prawns: an invertebrate virus with ORF1a and ORF1b genes related to arteri- and coronaviruses. J. Gen. Virol. 81:1473–1484 [DOI] [PubMed] [Google Scholar]
- 19. Schütze H, et al. 2006. Characterization of White bream virus reveals a novel genetic cluster of nidoviruses. J. Virol. 80:11598–11609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beaudette FR, Hudson CB. 1937. Cultivation of the virus of infectious bronchitis. J. Am. Vet. Med. Assoc. 90:51–58 [Google Scholar]
- 21. Poon LL, et al. 2005. Identification of a novel coronavirus in bats. J. Virol. 79:2001–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang XC, et al. 2006. Prevalence and genetic diversity of coronaviruses in bats from China. J. Virol. 80:7481–7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woo PC, Lau SK, Yuen KY. 2006. Infectious diseases emerging from Chinese wet-markets: zoonotic origins of severe respiratory viral infections. Curr. Opin. Infect. Dis. 19:401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cowley JA, Dimmock CM, Walker PJ. 2002. Gill-associated nidovirus of Penaeus monodon prawns transcribes 3′-coterminal subgenomic mRNAs that do not possess 5′-leader sequences. J. Gen. Virol. 83:927–935 [DOI] [PubMed] [Google Scholar]
- 25. Gorbalenya AE, Enjuanes L, Ziebuhr J, Snijder EJ. 2006. Nidovirales: evolving the largest RNA virus genome. Virus Res. 117:17–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasternak AO, Spaan WJ, Snijder EJ. 2006. Nidovirus transcription: how to make sense...? J. Gen. Virol. 87:1403–1421 [DOI] [PubMed] [Google Scholar]
- 27. Sittidilokratna N, Dangtip S, Cowley JA, Walker PJ. 2008. RNA transcription analysis and completion of the genome sequence of yellow head nidovirus. Virus Res. 136:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gosert R, Kanjanahaluethai A, Egger D, Bienz K, Baker SC. 2002. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 76:3697–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knoops K, et al. 2008. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 6:e226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schlegel A, Giddings TH, Jr, Ladinsky MS, Kirkegaard K. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 70:6576–6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ziebuhr J, Snijder EJ, Gorbalenya AE. 2000. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 81:853–879 [DOI] [PubMed] [Google Scholar]
- 32. Brierley I, Dos Ramos FJ. 2006. Programmed ribosomal frameshifting in HIV-1 and the SARS- CoV. Virus Res. 119:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Namy O, Moran SJ, Stuart DI, Gilbert RJ, Brierley I. 2006. A mechanical explanation of RNA pseudoknot function in programmed ribosomal frameshifting. Nature 441:244–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng A, et al. 2005. Expression, purification, and characterization of SARS coronavirus RNA polymerase. Virology 335:165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhardwaj K, Guarino L, Kao CC. 2004. The severe acute respiratory syndrome coronavirus Nsp15 protein is an endoribonuclease that prefers manganese as a cofactor. J. Virol. 78:12218–12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ivanov KA, et al. 2004. Major genetic marker of nidoviruses encodes a replicative endoribonuclease. Proc. Natl. Acad. Sci. U. S. A. 101:12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minskaia E, et al. 2006. Discovery of an RNA virus 3′->5′ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc. Natl. Acad. Sci. U. S. A. 103:5108–5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Snijder EJ, et al. 2003. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 331:991–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Grotthuss M, Wyrwicz LS, Rychlewski L. 2003. mRNA cap-1 methyltransferase in the SARS genome. Cell 113:701–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Putics A, Filipowicz W, Hall J, Gorbalenya AE, Ziebuhr J. 2005. ADP-ribose-1"-monophosphatase: a conserved coronavirus enzyme that is dispensable for viral replication in tissue culture. J. Virol. 79:12721–12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saikatendu KS, et al. 2005. Structural basis of severe acute respiratory syndrome coronavirus ADP-ribose-1''-phosphate dephosphorylation by a conserved domain of nsP3. Structure 13:1665–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brierley I. 1995. Ribosomal frameshifting viral RNAs. J. Gen. Virol. 76(Pt. 8):1885–1892 [DOI] [PubMed] [Google Scholar]
- 43. Brierley I, Jenner AJ, Inglis SC. 1992. Mutational analysis of the “slippery-sequence” component of a coronavirus ribosomal frameshifting signal. J. Mol. Biol. 227:463–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacks T, Madhani HD, Masiarz FR, Varmus HE. 1988. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell 55:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim KH, Lommel SA. 1994. Identification and analysis of the site of −1 ribosomal frameshifting in red clover necrotic mosaic virus. Virology 200:574–582 [DOI] [PubMed] [Google Scholar]
- 46. Kim KH, Lommel SA. 1998. Sequence element required for efficient −1 ribosomal frameshifting in red clover necrotic mosaic dianthovirus. Virology 250:50–59 [DOI] [PubMed] [Google Scholar]
- 47. Sawicki SG, Sawicki DL. 1995. Coronaviruses use discontinuous extension for synthesis of subgenome-length negative strands. Adv. Exp. Med. Biol. 380:499–506 [DOI] [PubMed] [Google Scholar]
- 48. van der Most RG, Luytjes W, Rutjes S, Spaan WJ. 1995. Translation but not the encoded sequence is essential for the efficient propagation of the defective interfering RNAs of the coronavirus mouse hepatitis virus. J. Virol. 69:3744–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lai MM, Cavanagh D. 1997. The molecular biology of coronaviruses. Adv. Virus Res. 48:1–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Snijder EJ, Meulenberg JJ. 1998. The molecular biology of arteriviruses. J. Gen. Virol. 79(Pt. 5):961–979 [DOI] [PubMed] [Google Scholar]
- 51. van Vliet AL, Smits SL, Rottier PJ, de Groot RJ. 2002. Discontinuous and non-discontinuous subgenomic RNA transcription in a nidovirus. EMBO J. 21:6571–6580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cowley JA, Cadogan LC, Spann KM, Sittidilokratna N, Walker PJ. 2004. The gene encoding the nucleocapsid protein of Gill-associated nidovirus of Penaeus monodon prawns is located upstream of the glycoprotein gene. J. Virol. 78:8935–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jendrach M, Thiel V, Siddell S. 1999. Characterization of an internal ribosome entry site within mRNA 5 of murine hepatitis virus. Arch. Virol. 144:921–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu DX, Inglis SC. 1992. Internal entry of ribosomes on a tricistronic mRNA encoded by infectious bronchitis virus. J. Virol. 66:6143–6154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O’Connor JB, Brian DA. 2000. Downstream ribosomal entry for translation of coronavirus TGEV gene 3b. Virology 269:172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thiel V, Siddell SG. 1994. Internal ribosome entry in the coding region of murine hepatitis virus mRNA 5. J. Gen. Virol. 75(Pt. 11):3041–3046 [DOI] [PubMed] [Google Scholar]
- 57. Vijaykrishna D, et al. 2007. Evolutionary insights into the ecology of coronaviruses. J. Virol. 81:4012–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Woo PC, Lau SK, Huang Y, Yuen KY. 2009. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. (Maywood) 234:1117–1127 [DOI] [PubMed] [Google Scholar]
- 59. Pignatelli J, et al. 2009. Molecular characterization of a new PToV strain. Evolutionary implications. Virus Res. 143:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Johnson PT, Thieltges DW. 2010. Diversity, decoys and the dilution effect: how ecological communities affect disease risk. J. Exp. Biol. 213:961–970 [DOI] [PubMed] [Google Scholar]
- 61. Ostfeld RS. 2009. Biodiversity loss and the rise of zoonotic pathogens. Clin. Microbiol. Infect. 15(Suppl. 1):40–43 [DOI] [PubMed] [Google Scholar]
- 62. Biel SS, Gelderblom HR. 1999. Electron microscopy of viruses. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 63. Hayat MA. 2000. Principles and techniques of electron microscopy: biological applications, 4th ed Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 64. Stang A, Korn K, Wildner O, Uberla K. 2005. Characterization of virus isolates by particle-associated nucleic acid PCR. J. Clin. Microbiol. 43:716–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tamura K, et al. 4 May 2011, posting date. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. [Epub ahead of print.] doi: 10.1093/molbev/msr121 [Epub ahead of print.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Price MN, Dehal PS, Arkin AP. 2010. FastTree 2─approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple sequence alignments of putative CAVV replicative proteins. (A) Alignment of astro-, poty-, and nidovirus 3C-like protease domains. Putative catalytic Cys, His, and Asp/Glu residues are indicated by asterisks. Also indicated are conserved residues predicted to be part of the S1 specificity subsite (&). (B) Alignment of CAVV and coronavirus helicase-associated N-terminal ZBDs. Putative zinc-binding residues are indicated (#). (C) Alignment of nidoviral endoribonuclease (NendoU) domains. Putative catalytic residues (His, His, Lys) are indicated by asterisks. Partial pp1a/pp1ab sequences were aligned using ClustalX 2.0 (Larkin MA, et al., Bioinformatics 23:2947–2948, 2007) and rendered with ESPript 2.2 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi) (Gouet P, Courcelle E, Stuart DI, Metoz F, Bioinformatics 15:305–308, 1999). Abbreviations of virus names and accession numbers are as follows: BToV, bovine torovirus (strain Breda 1) (AY427798); EToV, equine torovirus (strain Berne) (X52374); WBV, white bream virus (strain DF24/00) (NC_008516); EAV, equine arteritis virus (strain Bucyrus) (NP_127506); PRRSV, porcine reproductive and respiratory syndrome virus (strain Lelystad) (Q04561); LDV, lactate dehydrogenase-elevating virus (neurovirulent type C) (Q06502); GAV, gill-associated virus (AF227196); TEV, tobacco etch virus (AAA47909); HAstV-1, human astrovirus 1 (AAW51880); HCoV-229E, human coronavirus 229E (NC_002645); HCoV-NL63, human coronavirus NL63 (NC_005831); TGEV, transmissible gastroenteritis virus (strain Purdue-115) (Z34093); MHV-A59, murine hepatitis virus (strain A59) (NC_001846); SARS-CoV, severe acute respiratory syndrome-related CoV (strain Frankfurt-1) (AY291315); HCoV-OC43, human CoV strain OC43 (AY585229); IBV, avian infectious peritonitis virus (strain Beaudette) (NC_001451). Download Figure S1, PDF file, 0.420 MB.
CAVV leader-to-body fusion sites. Leader-to-body fusion sites of CAVV subgenomic mRNAs were identified using the SuperScript III One-Step RT-PCR System with Platinum Taq High Fidelity (Invitrogen, Karlsruhe, Germany) with leader-specific primers in combination with subgenomic-mRNA-specific reverse primers (primers are indicated by arrowheads) (see Table S1). Primers that yielded no PCR products are shown in grey. PCR products were sequenced and, in the case of unclear sequencing results, TA cloned into the pCR2.1 TOPO vector (Invitrogen, Karlsruhe, Germany) and resequenced. Download Figure S2, JPG file, 0.315 MB.
CAVV primers used in this study.