Figure 4.
Membrane Protein Stability Is Predominantly Intrinsic and Is Related to Its Propensity to Form Well-Ordered Crystals
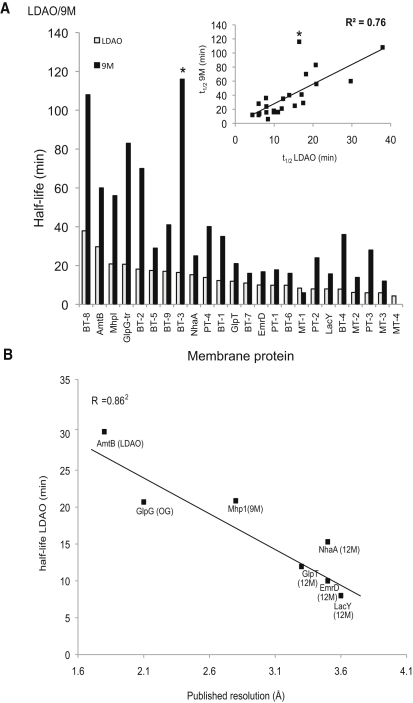
(A) Bars represent the unfolding half-life for each protein in 9M (filled) and plotted against that measured in LDAO (nonfilled); unfolding rates for LDAO were plotted from the highest to lowest (left to right). Inset is a linear curve indicating the average stability difference between LDAO and NM. Asterisk for protein BT-3 indicates that we considered this difference as an outlier and, as such, was not included in calculation of the correlation coefficient as displayed in inset.
(B) Membrane protein stability, as judged by unfolding rates in the detergent LDAO, correlates to the published resolution of control membrane proteins. The detergent used for crystallization is listed in brackets besides each protein. See also Figure S8.