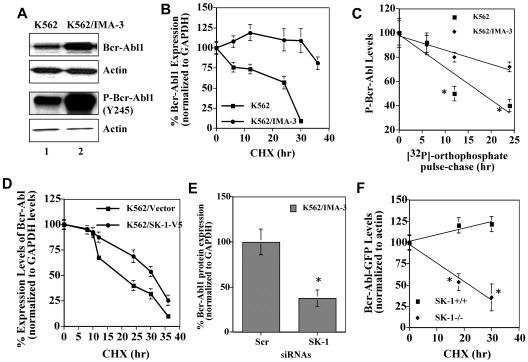
Figure 1.
Regulation of the stability and expression of Bcr-Abl1 by SK-1/S1P signaling. (A) Total and P-Bcr-Abl1 (Y245) in K562/IMA-3 compared with K562 cells (lanes 2 and 1, respectively) were measured using Western blotting. β-actin was used as a loading control. (B) The protein stability of Bcr-Abl1 in K562 and K562/IMA-3 cells was measured by Western blotting at various time points (0-36 hours) using CHX. Long-lived protein GAPDH was used as a control. Relative expression levels of Bcr-Abl1 (normalized to GAPDH levels) at 0-36 hours after CHX treatment in K562 versus K562/IMA-3 cells are shown. (C) The stability of P-Bcr-Abl1 in K562 and K562/IMA-3 cells were also examined by pulse-chase experiments using [32P]-orthophosphate labeling without CHX using immunoprecipitation by anti–P-Bcr-Abl1 (Y177) antibody. (D) The protein stability of Bcr-Abl1 in response to overexpression of SK-1 with the V5 tag in K562 parental cells was determined at various time points (0-36 hours) compared with vector-transfected controls using Western blotting. GAPDH was used as a loading control (left panel). (E) The expression of SK-1 was down-regulated using siRNA, and its effect on the stability of Bcr-Abl1 was determined using CHX treatment at 12 hours compared with Scr-siRNA treated controls. (F) Effects of genetic loss of SK-1 on Bcr-Abl1 stability were examined in wt and SK-1−/− MEFs stably transfected with the human wt-Bcr-Abl1-GFP (p210) in the presence of CHX at 0, 18, 30 hours. The stability of P-Bcr-Abl1-GFP in wt versus SK-1−/− MEFs was measured using [32P]-orthophosphate labeling and immunoprecipitation (using the antibody that recognizes P-Bcr-Abl1 at Y177) as described in “Determining the half-life of Bcr-Abl1 protein.” These experiments were performed in duplicates, and error bars represent SD. *P < .05 was considered significant.