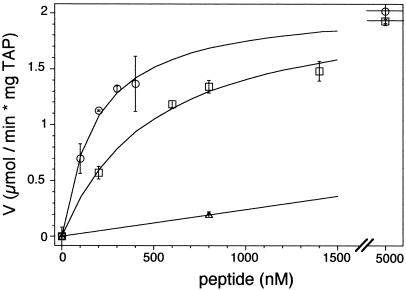
Figure 4.
Coupling between peptide binding and ATP hydrolysis. Release of γ-32Pi from [γ-32P]ATP was analyzed after 4 min at 32°C in the presence of various peptides. RRYQKSTEL (circles), RRYNASTEL (squares), and EPGNTWDED (triangles) have high, intermediate, and low binding affinity for TAP, respectively. Half-maximal stimulation (Km,pep) was obtained at 161 ± 15 nM and 574 ± 132 nM for RRYQKSTEL and RRYNASTEL, respectively. For the peptide with low affinity, only a lower limit of the half-maximal ATPase stimulation could be given. An identical maximal peptide-stimulated ATPase activity (Vmax) of 2 μmol/min per milligram TAP was found for RRYQKSTEL and RRYNASTEL. Data were obtained from duplicate measurements.