Figure 4.
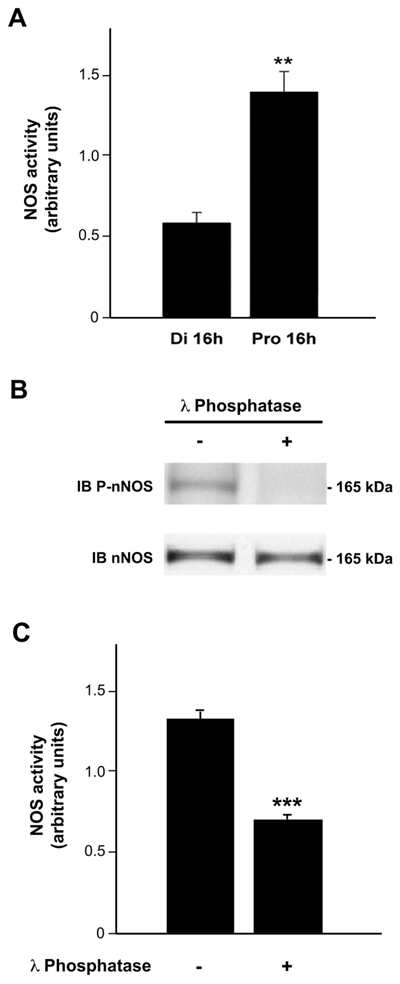
λ-phosphatase-mediated dephosphorylation of nNOS at Ser1412 impairs NOS activity in protein extracts from the preoptic region of the hypothalamus. A. Representative bargraph of NOS activity in homogenized preoptic region fragments from diestrus (n= 4) or proestrus (n= 5) female rats. NOS activity was determined by measuring the formation of nitrite produced in samples. Nitrite assays indicated that preoptic explants had a significantly increased NOS activity on proestrus compared to diestrus. B. Proteins extracts of the preoptic region from female rats in proestrus were first dephosphorylated or not (+ or -) by using λ-phosphatase. Samples were then submitted to electrophoresis and Western blotting using three different antibodies (anti-P-nNOS, anti-nNOS and anti-PSD95). Dephosphorylation of proteins with λ-phosphatase resulted in a complete abolishment of PnNOS signal, whereas no effect on nNOS expression was observed. C. The same experiments were repeated to determine whether dephosphorylation of nNOS affects NOS activity (n = 4 per group). The graph illustrates the significant decrease in NOS activity after dephosphorylation. Error bars indicate SEM. ** p < 0.01; *** p < 0.001.
