Figure 7.
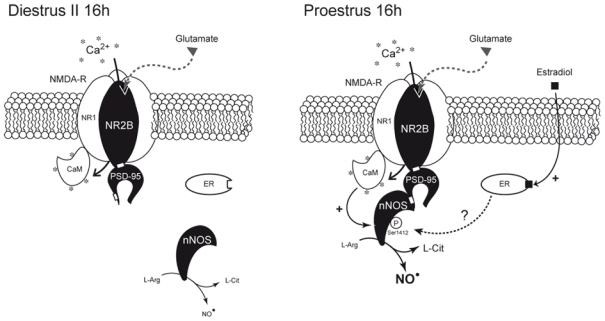
Schematic representation of the possible estradiol-mediated changes in proteinprotein interactions involved in the control of nNOS activity in the preoptic region of the hypothalamus during the ovarian cycle. Neuronal NOS activity is primarily regulated by increases in the local intracellular [Ca2+] (*), which activates nNOS through calmodulin (CaM) binding (29). The physical interaction of nNOS with NMDA receptors involves the postsynaptic density-95 (PSD-95) scaffolding protein and the assembly of a ternary complex (32). Only Ca2+ influx through the NMDA receptor promotes an efficient NO production (29, 30). In parallel, nNOS is also subjected to posttranscriptional modifications (such as phoshorylation) that modulates its catalytic activity (36–39). Natural fluctuations of estrogen levels across the ovarian cycle, i.e., low in diestrus (Di 16h) and high in proestrus (Pro 16h), regulate the activation state of nNOS by modulating its coupling with NR2B-containing NMDA receptors by the PSD-95 scaffolding protein. In turn, this regulation, that could be mediated by estrogen-dependent estrogen receptor (ER) activation (62), results in the phosphorylation of nNOS, an effect known to increase nNOS enzymatic activity (37, 65).
