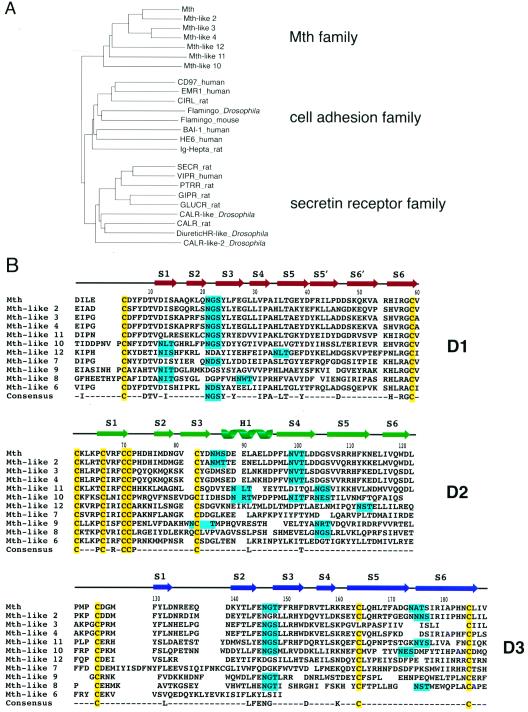
Figure 1.
Relation of Mth to family B GPCRs and other Mth homologs. (A) Phylogenetic relationship of transmembrane regions of GPCR family B proteins. A phylogenetic tree was generated by using the neighbor-joining method based on an alignment of transmembrane domains generated with the clustalw program (25). The human β-2 adrenergic receptor (not shown) served as the outgroup. A subfamily consisting of ≈10 Mth-like proteins identified from the Drosophila genome project clusters with Mth. CIRL, calcium-independent receptor of α-latrotoxin; BAI-1, brain-specific angiogenesis inhibitor 1; HE6, human epididymal gene product 6; SECR, secretin receptor; VIPR, vasoactive intestinal peptide receptor; PTRR, parathyroid hormone-related peptide receptor; GIPR, gastric inhibitory peptide receptor; GLUCR, glucagon receptor; CALR, calcitonin receptor. (B) Sequence alignment of the Mth ectodomain with predicted homologs from the Drosophila genome. Residue numbering begins at the first residue of the mature protein. Crystallographically determined secondary-structural elements are shown above the sequences. The 10 cysteines that form five disulfide bonds in the Mth ectodomain are highlighted in yellow, and potential N-linked glycosylation sites are highlighted in cyan.