Table 1.
Investigation of Scope in Heterocycle
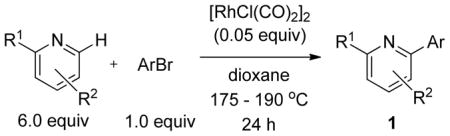 | ||||
|---|---|---|---|---|
| entry | azine | ArBr | product | yield (%)a |
| 1 | 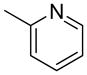 |
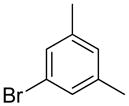 |
1a | 53 |
| 2 | 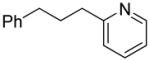 |
“ | 1b | 51 |
| 3 | 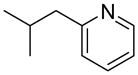 |
“ | 1c | 78 |
| 4 | 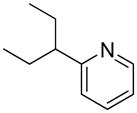 |
“ | 1d | 70 |
| 5 | 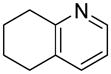 |
“ | 1e | 76 |
| 6 | 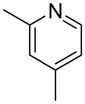 |
“ | 1f | 67 |
| 7 | 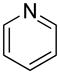 |
“ | 1g | 0 |
| 8 | 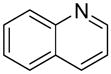 |
“ | 1h | 86b |
| 9 | 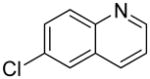 |
“ | 1i | 65b |
| 10 | 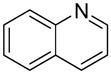 |
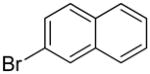 |
1j | 73b |
| 11 | 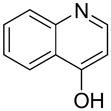 |
“ | 1k | 49c |
| 12 | 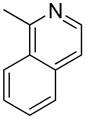 |
“ | 1l | 24b |
| 13 | 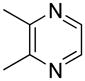 |
“ | 1m | 26b |
| 14 | 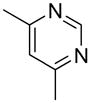 |
“ | 1n | 33b |
Isolated yield of analytically pure product; 0.4 mmol scale in ArBr; 0.8 M absolute concentration in ArBr;
Reaction run at 175 °C and 0.3 M absolute concentration in ArBr;
Reaction run at 165 °C and 0.3 M absolute concentration in ArBr.
