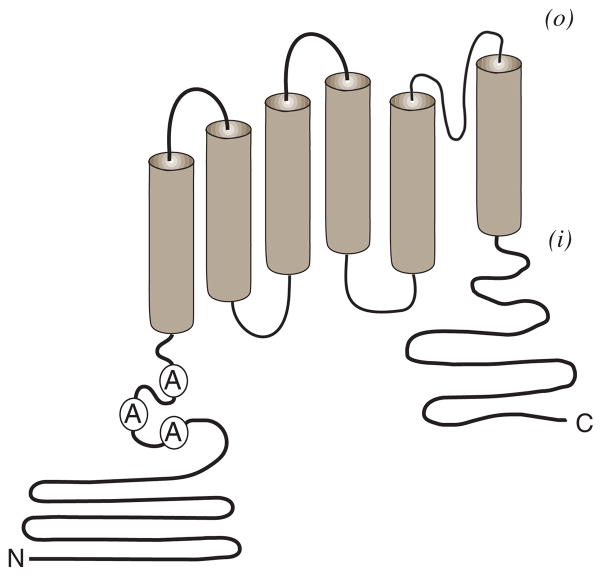
Figure 2. TRPV1 protein topology.
TRPV1 is distinguished by six transmembrane spanning regions flanked by two intracellular domains (N) amino-terminal and (C) carboxyl-terminal. The N-terminal domain includes three ankyrin (A) repeat domains that may function in receptor modulation. A pore loop domain is predicted between the fifth and sixth transmembrane spanning region. It is proposed that at least four such subunits assemble to form a functional channel complex. Formation of hetermomeric channel complexes incorporating TRPV1 plus other TRPV1 splice variant and/or TRP – channel subunits have been proposed.