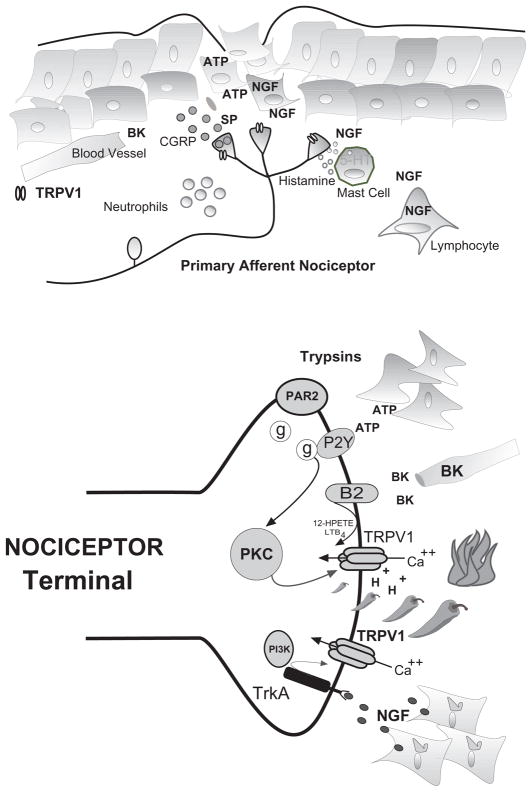
Figure 3. Mechanisms of TRPV1 mediated inflammatory hyperalgesia and pain transduction (top).
Following tissue injury, local tissue responds with increased production and accumulation of inflammatory compounds that activate/sensitize TRPV1. Nociceptive terminals derived from C- and A-delta fibers are interposed with skin fibroblasts, mast cells and the microvasculature. Following injury or inflammation terminals depolarize releasing neuropeptides substance-P (SP) and calcitonin gene related peptide (CGRP) which produces vascular leak and edema. Bradykinin (BK) cleaved from circulating kallikreins and nerve growth factor (NGF) produced by fibroblasts an infiltrating PBMs both activate and sensitize nociceptor terminals. NGF produces additional sensitization through the degranulation of mast cells containing serotonin (5-HT) and histamine. NGF and cytokines are associated with the accumulation of neutrophils and lymphocytes that participate in the maintenance of sensitization -hyperalgesia. (Bottom) Hypothetical nociceptor terminal expressing TRPV1 activated by capsaicin (peppers), noxious heat (fire) and extracellular protons (H+). The resulting inward calcium current depolarizes terminal initiating action potentials that signal higher centers (not shown). Inflammatory mediators such as bradykinin (BK), ATP, trypsins, and NGF act through various secondary messenger systems to activate or sensitize TRPV1, resulting in pain and hyperalgesia.