Abstract
Roles of calcineurin (CaN), a Ca2+/calmodulin- (CaM-) dependent protein phosphatase, and Ca2+/CaM-dependent protein kinase-II (CaMKII) in modulating K+ channel activity and the intracellular Ca2+ concentration ([Ca2+]i) have been investigated in renal tubule epithelial cells. The channel current through the cell membrane was recorded with the patch-clamp technique, and [Ca2+]i was monitored using fura-2 imaging. We found that a CaN-inhibitor, cyclosporin A (CyA), lowered the K+ channel activity and elevated [Ca2+]i, suggesting that CyA closes K+ channels and opens Ca2+-release channels of the cytosolic Ca2+-store. Moreover, both of these responses were blocked by KN-62, an inhibitor of CaMKII. It is suggested that the CyA-mediated response results from the activation of CaMKII. Indeed, Western blot analysis revealed that CyA increased phospho-CaMKII, an active form of CaMKII. These findings suggest that CaN-dependent dephosphorylation inhibits CaMKII-mediated phosphorylation, and the inhibition of CaN increases phospho-CaMKII, which results in the stimulation of CaMKII-dependent cellular actions.
1. Introduction
Protein kinases and phosphatases regulate major parts of functional proteins through the phosphorylation and dephosphorylation of individual proteins. The characterization and roles of many kinds of protein kinases and phosphatases have been widely investigated in a variety of cells.
Ion channels are well-known functional proteins and their activity can be estimated by monitoring ion currents using the patch-clamp technique. Since the activity of several kinds of channel protein is regulated by protein kinase-mediated phosphorylation and phosphatase-mediated dephosphorylation [1, 2], the investigation of regulatory mechanisms for ion channels often provides important findings regarding the functional significance of protein kinases and phosphatases. To date, it has been demonstrated that some serine/threonine protein kinases, such as protein kinase A (PKA), protein kinase G (PKG), and protein kinase C (PKC), affect ion channel activity. In renal tubule cells, PKA [3–5] and PKG stimulates, [6, 7] and PKC inhibits [3, 5] the activity of inwardly rectifying K+ channels. In such cases, it was suggested that the PKA- or PKG-mediated phosphorylation site was different from the PKC-mediated phosphorylation site, since the effects of PKA and PKG on the channel functions were different from those of PKC. Although the PKG-mediated site is still unknown, molecular analyses have revealed that PKA-mediated and PKC-mediated sites exist in channel proteins [8, 9]. As for the effect of protein phosphatase on the activity of K+ channels, protein phosphatase-1 (PP-1) [10] and protein phosphatase-2A (PP-2A) [10, 11] were reported to inhibit channel activity in renal tubule cells, suggesting that PKA-mediated phosphorylation was dephosphorylated by PP-1 and/or PP-2A.
It was also reported that the inhibitory effect on channel activity of PKC was mimicked by Ca2+/CaM kinase II (CaMKII) in renal tubule cells [12]. However, the phosphatase which induces dephosphorylation of PKC or the CaMKII site remains unknown. Recently, we discovered that calcineurin (CaN), a Ca2+/CaM-dependent protein phosphatase, had an opposite effect to CaMKII on channel activity in human renal tubule cells [12].
Based on the recent reports including our data [13], we have reviewed the functional significance and mutual effects of CaN and CaMKII on channel activity, as well as on modulation of the intracellular Ca2+ concentration ([Ca2+]i). We will further discuss the regulatory mechanisms controlling channel activity and [Ca2+]i by phosphorylation and dephosphorylation in cultured renal tubule cells, which we have used in recent studies [7, 10, 13].
2. Mutual Effects of Calcineurin (CaN) and CaMKII on K+ Channel Activity in Human Tubule Cells
CaN is a Ca2+-dependent protein phosphatase [14], often called protein phosphatase-2B (PP-2B) [15], and is known to be involved in some functions of various cells [15–17]. Since CaN induces the dephosphorylation of several phosphorylated protein serine/threonine residues [14, 17, 18], it is likely that phosphorylation induced by Ca2+-dependent protein kinase, such as PKC or CaMKII, is dephosphorylated by CaN.
It was demonstrated that cyclosporin A (CyA), an inhibitor of CaN, suppressed the activity of the inwardly rectifying K+ channels in renal tubule cells [19, 20]. We also found that inhibitors of CaN, CyA and FK520, both of which are well-known immunosuppressive agents [21], suppress K+ channel activity in cell-attached patches of tubule cells under normal conditions [19, 20]. Since inhibitors of CaN are effective under normal conditions, CaN would be functionally activated even in a normal [Ca2+]i. However, the mechanism of CyA-induced channel inhibition is still unknown. To examine the involvement of Ca2+-dependent protein kinase in CyA-induced K+ channel suppression, we applied a PKC inhibitor, GF109203X [22], and a CaMKII inhibitor, KN-62 [23], and observed their effects on CyA-induced channel suppression. As the results, the CyA-induced channel suppression was not affected by GF109203X, but significantly attenuated by KN-62 [13], suggesting that the CyA-induced channel inhibition is mediated mainly by CaMKII. Although PKC may have an inhibitory effect on the inwardly rectifying K+ channels in renal tubule cells [3, 5], our results suggest that the major candidate evoking CyA-induced channel inhibition is CaMKII [13]. Indeed, Western blot analysis revealed that CyA increased phospho-CaMKII, an active form of CaMKII [13].
The direct effects of CaN and CaMKII on channel activity were also examined in inside-out patches. Before investigation of the effects of CaN and CaMKII, we analyzed the direct effects of cytoplasmic Ca2+ and CaM on channel activity, since it was reported that the activity of some channels was directly affected by Ca2+ [24] or CaM [16]. We confirmed that Ca2+ (1 μM) or CaM (0.6 μM) barely affected the K+ channels from the inside of the cell membrane, at least under our experimental conditions [13]. Moreover, the application of CaN in the presence of Ca2+ and CaM also induced no appreciable change in channel activity [13]. These findings suggest that CaN has no inhibitory effect on channel activity. In contrast, CaMKII markedly suppressed the channel activity. Then, we tested the direct effect of CaN on channel activity in inside-out patches in the presence of Ca2+, CaM, and CaMKII. The suppressed channel activity due to CaMKII was restored following the application of CaN [13]. These results suggest that CaMKII-mediated phosphorylation has an inhibitory effect on channel activity, and CaN reactivates channels by the dephosphorylation of CaMKII-mediated phosphorylation sites. However, the phosphorylation and dephosphorylation sites of kinases and phosphatases are still unclear. It is also possible that CaN induces the dephosphorylation of phospho-CaMKII, resulting in the inhibition of CaMKII.
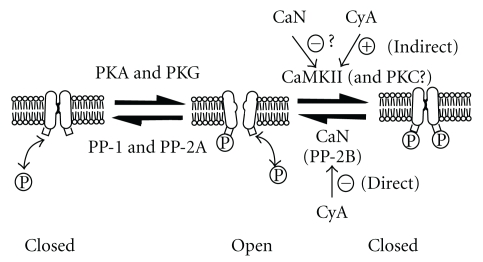
A schematic representation of the mechanism of K+ channel regulation by phosphorylation and dephosphorylation is shown in Figure 1. Previously, it was reported that PKA- and PKG-mediated phosphorylation induced channel opening (an active state) in proximal tubule cells [4–6] and that the open channels were closed by PP-1 and PP-2A [10], suggesting that PKA- or PKG-mediated phosphorylation was dephosphorylated by PP-1 or PP-2A. In addition to the above data, our findings strongly suggest that CaMKII phosphorylates other sites, resulting in the closed state of channels. On the other hand, CaN induces channel opening by the dephosphorylation of the CaMKII-mediated phosphorylation site or may inhibit CaMKII by the dephosphorylation of phospho-CaMKII. CyA directly inhibits CaN and indirectly increases the active type of CaMKII, phospho-CaMKII, through inhibition of the dephosphorylation process by CyA.
Figure 1.
A schematic representation of the model for the regulation of the inwardly rectifying K+ channels in renal tubule cells by phosphorylation and dephosphorylation processes. CaN is often called protein phosphatase-2B (PP-2B). The circled “P” indicates phosphate. Circled “+” and “−” indicate stimulation and inhibition, respectively. Small squares attached to the lower part of the channel are putative phosphorylation sites. See text for details.
3. Role of CaN and CaMKII in Modulation of [Ca2+] i in Human Tubule Cells
Our data on [Ca2+]i measurement showed that both CyA and FK520, inhibitors of CaN, elevated [Ca2+]i [13], similar to the data reported previously [25, 26]. Thus, it is suggested that CaN has an inhibitory effect on Ca2+-release from the intracellular Ca2+-store. As mentioned above, CyA-induced K+channel suppression results from stimulation of CaMKII-dependent processes. Thus, CaMKII may be a key factor for CaN inhibitor-mediated [Ca2+]i elevation in the tubule cells. The following experiments with inhibitors of both CaMKII and CaN revealed that CyA-induced [Ca2+]i elevation was blocked by KN-62 [13], suggesting the importance of CaMKII in the elevation of [Ca2+]i. Similar data were demonstrated in a previous report showing that CaMKII stimulates Ca2+ release from Ca2+-stores in skeletal muscle [27].
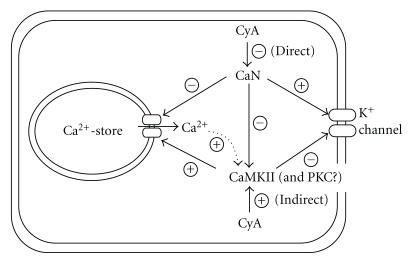
Figure 2 shows a simplified putative model of the mechanisms regarding Ca2+-release channels in intracellular Ca2+-stores and K+ channels in the cell membrane by CaN and CaMKII. CaN activates the K+ channel in the cell membrane and inhibits the Ca2+-release channels of intracellular Ca2+-stores. However, CaMKII inhibits K+ channels and activates Ca2+-release channels. Although there is a report of the role of CaN in the stimulation of Ca2+ flux via the 1,4,5-triphosphate receptor [28], our data strongly suggest that the inhibition of CaN stimulates Ca2+-release channels [13]. Moreover as mentioned above, the inhibition of CaN-mediated dephosphorylation by CyA increases phospho-CaMKII, an active form of CaMKII. Thus, it is suggested that [Ca2+]i elevation by CyA is induced mainly by increased phospho-CaMKII through activation of the Ca2+-release channels of the intracellular Ca2+-store. It is also conceivable that CaN exhibits an inhibitory effect on CaMKII, probably by the dephosphorylation of the phospho-CaMKII. The elevated [Ca2+]i may further stimulate the activity of CaMKII, which would enhance the suppression of K+ channel activity, as shown by the dotted curve in Figure 2. Moreover, the elevated [Ca2+]i may stimulate not only CaMKII but also PKC.
Figure 2.
A simplified model of the involvement of CaN and CaMKII in the modulation of K+ channel activity and Ca2+-release channels in intracellular Ca2+-stores in renal tubule cells. Circled “+” and “−” indicate stimulation and inhibition, respectively. Actions of CyA on Ca2+-release channel and CaMKII are also shown. Ca2+ released from the Ca2+-store may further enhance the activity of CaMKII, as shown by the dotted line.
4. Functional Relationship between CaN and CaMKII
Previous reports have shown that CaN plays several key roles in cellular functions. As for Ca2+-release from intracellular stores, CaN is directly involved in its regulation [26], or CaN inhibitors lead to a higher probability of the ryanodine receptor (RyR)/Ca2+-release channels being open [25]. On the other hand, CaMKII reportedly stimulates the Ca2+-release in skeletal muscle cells [27]. Thus, it is likely that the CaMKII-mediated stimulation of Ca2+-release was similar to the enhanced Ca2+-release induced by the inhibition of CaN, as observed in our study [13]. These reports support our experimental data that CaN and CaMKII have opposite effects on channel activity. However, it has been reported that target phosphorylation sites dephosphorylated by CaN are mainly mediated by PKA [29]. Indeed, the functional coupling of CaN and PKA was shown to modulate Ca2+-release in ventricular myocytes [29]. It has also been demonstrated that Na+/K+ ATPase at the basolateral membrane of kidney tubular epithelia was inhibited by CaN [30] and stimulated by PKA [31]. On the other hand, a cardiac Na+/Ca2+ exchanger was reported to be regulated by CaN and PKC [32]. A cellular process dependent on mitogen-activated protein kinase was reported to be negatively regulated by CaN [33]. Thus, protein kinases opposed to CaN-mediated processes would act not in unity. Only a few reports suggested that CaMKII-mediated processes were abolished by CaN [34, 35]. Our data indicate the possibility that CaMKII-mediated phosphorylation is blocked by CaN [13], although it is still unclear whether the CaMKII-mediated phosphorylation site is identical to the CaN-mediated dephosphorylation site. It is also conceivable that the target dephosphorylation site for CaN is the phosphorylation site of CaMKII. In such a case, CaN would directly inhibit CaMKII activity by the dephosphorylation of phospho-CaMKII.
Our data suggest that CaN-mediated protein dephosphorylation would be dominant compared to CaMKII-mediated phosphorylation under normal conditions, since CaN inhibitors were found to significantly suppress K+ channel activity, but an inhibitor of CaMKII alone did not [13]. Although the involvement of CaN in the modulation of channel activity is variable [36–39], our data suggest that the maintenance of channel opening under normal conditions would require the CaN-mediated action to exceed the CaMKII-mediated action in the tubule cells used in our study. This notion was supported by a previous report that the CaN content in proximal tubule cells is high among the several nephron segments [40]. However, it is still unknown whether the site of CaN-mediated dephosphorylation is identical to that of CaMKII-mediated phosphorylation in the modulation of channel activity. Moreover, there remains a possibility that the elevation of [Ca2+]i may be dependent on the entry of extracellular Ca2+ across the cell membrane [41]. Recently we also demonstrated Ca2+ entry across the cell membrane in renal tubule cells [42], although a CyA-induced [Ca2+]i elevation is considered to mainly be mediated by Ca2+-release channels in the membranes of intracellular Ca2+-stores. Further studies are necessary to clarify the precise mechanisms for the molecular regulation of channel activity as well as [Ca2+]i by protein kinases and phosphatase, such as CaMKII and CaN.
Acknowledgment
This work was supported in part by a Grant from the Corporation for Private Schools of Japan (to M. kubokawa in 2010).
References
- 1.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annual Review of Physiology. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 2.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiological Reviews. 2005;85(1):319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang WH, Giebisch G. Dual modulation of renal ATP-sensitive K+ channel by protein kinases A and C. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(21):9722–9725. doi: 10.1073/pnas.88.21.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubokawa M, Mori Y, Kubota T. Modulation of inwardly rectifying ATP-regulated K+ channel by phosphorylation process in opossum kidney cells. Japanese Journal of Physiology. 1997;47(1):111–119. doi: 10.2170/jjphysiol.47.111. [DOI] [PubMed] [Google Scholar]
- 5.Mauerer UR, Boulpaep EL, Segal AS. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. Journal of General Physiology. 1998;111(1):161–180. doi: 10.1085/jgp.111.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubokawa M, Nakaya S, Yoshioka Y, et al. Activation of inwardly rectifying K+ channel in OK proximal tubule cells involves cGMP-dependent phosphorylation process. Japanese Journal of Physiology. 1998;48(6):467–476. doi: 10.2170/jjphysiol.48.467. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, Hirano J, Itazawa S, Kubokawa M. Protein kinase G activates inwardly rectifying K+ channel in cultured human proximal tubule cells. American Journal of Physiology—Renal Physiology. 2002;283(4):F784–F791. doi: 10.1152/ajprenal.00023.2002. [DOI] [PubMed] [Google Scholar]
- 8.Ho K, Nichols CG, Lederer WJ, et al. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Hebert SC, Giebisch G. Renal K+ channels: structure and function. Annual Review of Physiology. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 10.Kubokawa M, Nakamura K, Hirano J, et al. Regulation of inwardly rectifying K+ channel in cultured opossum proximal tubule cells by protein phosphatases 1 and 2A. Japanese Journal of Physiology. 2000;50(2):249–256. doi: 10.2170/jjphysiol.50.249. [DOI] [PubMed] [Google Scholar]
- 11.Kubokawa M, McNicholas CM, Higgins HA, Wang W, Giebisch G. Regulation of ATP-sensitive K+ channel by membrane-bound protein phosphatases in rat principal tubule cell. American Journal of Physiology—Renal Physiology. 1995;269(3):F355–F362. doi: 10.1152/ajprenal.1995.269.3.F355. [DOI] [PubMed] [Google Scholar]
- 12.Kubokawa M, Wang W, McNicholas CM, Giebisch G. Role of Ca2+/CaMK II in Ca2+-induced K+ channel inhibition in rat CCD principal cell. American Journal of Physiology—Renal Physiology. 1995;268(2):F211–F219. doi: 10.1152/ajprenal.1995.268.2.F211. [DOI] [PubMed] [Google Scholar]
- 13.Kubokawa M, Kojo T, Komagiri Y, Nakamura K. Role of calcineurin-mediated dephosphorylation in modulation of an inwardly rectifying K+ channel in human proximal tubule cells. Journal of Membrane Biology. 2009;231(2-3):79–92. doi: 10.1007/s00232-009-9207-z. [DOI] [PubMed] [Google Scholar]
- 14.Rusnak F, Mertz F. Calcineurin: form and function. Physiological Reviews. 2000;80(4):1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature. 1994;369(6477):235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- 16.Rycroft BK, Gibb AJ. Inhibitory interactions of calcineurin (phosphatase 2B) and calmodulin on rat hippocampal NMDA receptors. Neuropharmacology. 2004;47(4):505–514. doi: 10.1016/j.neuropharm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Chin ER, Olson EN, Richardson JA, et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes and Development. 1998;12(16):2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donella-Deana A, Krinks MH, Ruzzene M, Klee C, Pinna LA. Dephosphorylation of phosphopeptides by calcineurin (protein phosphatase 2B) European Journal of Biochemistry. 1994;219(1-2):109–117. doi: 10.1111/j.1432-1033.1994.tb19920.x. [DOI] [PubMed] [Google Scholar]
- 19.Ling BN, Eaton DC. Cyclosporin A inhibits apical secretory K+ channels in rabbit cortical collecting tubule principal cells. Kidney International. 1993;44(5):974–984. doi: 10.1038/ki.1993.339. [DOI] [PubMed] [Google Scholar]
- 20.Ye B, Liu Y, Zhang Y. Properties of a potassium channel in the basolateral membrane of renal proximal convoluted tubule and the effect of cyclosporine on it. Physiological Research. 2006;55(6):617–622. doi: 10.33549/physiolres.930866. [DOI] [PubMed] [Google Scholar]
- 21.Jain A, Khanna A, Molmenti EP, Rishi N, Fung JJ. Immunosuppressive therapy. Surgical Clinics of North America. 1999;79(1):59–76. doi: 10.1016/s0039-6109(05)70007-4. [DOI] [PubMed] [Google Scholar]
- 22.Angulo J, Cuevas P, Fernández A, et al. Enhanced thromboxane receptor-mediated responses and impaired endothelium-dependent relaxation in human corpus cavernosum from diabetic impotent men: role of protein kinase C activity. Journal of Pharmacology and Experimental Therapeutics. 2006;319(2):783–789. doi: 10.1124/jpet.106.108597. [DOI] [PubMed] [Google Scholar]
- 23.Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. Journal of Biological Chemistry. 1990;265(8):4315–4320. [PubMed] [Google Scholar]
- 24.Mauerer UR, Boulpaep EL, Segal AS. Properties of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. Journal of General Physiology. 1998;111(1):139–160. doi: 10.1085/jgp.111.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandyopadhyay A, Shin DW, Ahn JO, Kim DH. Calcineurin regulates ryanodine receptor/Ca2+-release channels in rat heart. Biochemical Journal. 2000;352(1):61–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Bultynck G, Vermassen E, Szlufcik K, et al. Calcineurin and intracellular Ca2+-release channels: regulation or association? Biochemical and Biophysical Research Communications. 2003;311(4):1181–1193. doi: 10.1016/j.bbrc.2003.08.084. [DOI] [PubMed] [Google Scholar]
- 27.Tavi P, Allen DG, Niemelä P, Vuolteenaho O, Weckström M, Westerblad H. Calmodulin kinase modulates Ca2+ release in mouse skeletal muscle. Journal of Physiology. 2003;551(1):5–12. doi: 10.1113/jphysiol.2003.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor- FKBP12 complex modulates Ca2+ flux. Cell. 1995;83(3):463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 29.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. Journal of Physiology. 2002;544(1):57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumlin JA, Sands JM. Nephron segment-specific inhibition of Na+/K+-ATPase activity by cyclosporin A. Kidney International. 1993;43(1):246–251. doi: 10.1038/ki.1993.38. [DOI] [PubMed] [Google Scholar]
- 31.Carranza ML, Rousselot M, Chibalin AW, Bertorello AM, Favre H, Féraille E. Protein kinase A induces recruitment of active Na+, K+-ATPase units to the plasma membrane of rat proximal convoluted tubule cells. Journal of Physiology. 1998;511(1):235–243. doi: 10.1111/j.1469-7793.1998.235bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shigekawa M, Katanosaka Y, Wakabayashi S. Regulation of the cardiac Na+/Ca2+ exchanger by calcineurin and protein kinase C. Annals of the New York Academy of Sciences. 2007;1099:53–63. doi: 10.1196/annals.1387.059. [DOI] [PubMed] [Google Scholar]
- 33.Tian J, Karin M. Stimulation of Elk1 transcriptional activity by mitogen-activated protein kinases is negatively regulated by protein phosphatase 2B (calcineurin) Journal of Biological Chemistry. 1999;274(21):15173–15180. doi: 10.1074/jbc.274.21.15173. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Kanatous SB, Thurmond FA, et al. Regulation of mitochondrial biogenesis in skeletal muscle by caMK. Science. 2002;296(5566):349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 35.Gerges NZ, Alzoubi KH, Alkadhi KA. Role of phosphorylated CaMKII and calcineurin in the differential effect of hypothyroidism on LTP of CA1 and dentate gyrus. Hippocampus. 2005;15(4):480–490. doi: 10.1002/hipo.20073. [DOI] [PubMed] [Google Scholar]
- 36.Czirják G, Tóth ZE, Enyedi P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. Journal of Biological Chemistry. 2004;279(18):18550–18558. doi: 10.1074/jbc.M312229200. [DOI] [PubMed] [Google Scholar]
- 37.Loane DJ, Hicks GA, Perrino BA, Marrion NV. Inhibition of BKCa channel activity by association with calcineurin in rat brain. European Journal of Neuroscience. 2006;24(2):433–441. doi: 10.1111/j.1460-9568.2006.04931.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Lin DH, Wang ZJ, Jin Y, Yang B, Wang WH. K restriction inhibits protein phosphatase 2B (PP2B) and suppression of PP2B decreases ROMK channel activity in the CCD. American Journal of Physiology—Cell Physiology. 2008;294(3):C765–C773. doi: 10.1152/ajpcell.00528.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Zhang ZR, Chou CF, Liang YY, Gu Y, Ma HF. Cyclosporine stimulates the renal epithelial sodium channel by elevating cholesterol. American Journal of Physiology—Renal Physiology. 2009;296(2):F284–F290. doi: 10.1152/ajprenal.90647.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumlin JA. Expression and function of calcineurin in the mammalian nephron: physiological roles, receptor signaling, and ion transport. American Journal of Kidney Diseases. 1997;30(6):884–895. doi: 10.1016/s0272-6386(97)90100-1. [DOI] [PubMed] [Google Scholar]
- 41.Burley JR, Sihra TS. A modulatory role for protein phosphatase 2B (calcineurin) in the regulation of Ca2+ entry. European Journal of Neuroscience. 2000;12(8):2881–2891. doi: 10.1046/j.1460-9568.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 42.Komagiri Y, Nakamura K, Kubokawa M. A nicardipine-sensitive Ca2+ entry contributes to the hypotonicity-induced increase in [Ca2+]i of principal cells in rat cortical collecting duct. Cell Calcium. 2011;49(1):35–42. doi: 10.1016/j.ceca.2010.11.006. [DOI] [PubMed] [Google Scholar]