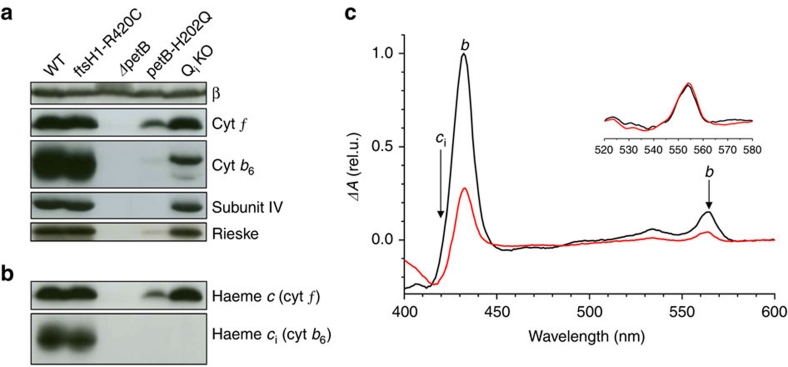
Figure 2. Characterization of cytochrome b6f complex in the QiKO strain.
(a) Immunoblot chemiluminescence analysis of the major subunits of cytochrome b6f. Subunit β of chloroplast ATPase as a loading control. QiKO shows wild-type level of all cytochrome b6f subunits with the doublet signature for cytochrome b6 missing the ci haem after SDS-urea PAGE. (b) Covalent haem peroxidasic activity confirms the absence of the ci haem in QiKO strain. (c) Dithionite minus ascorbate spectra from purified b6f complexes. Black, WT; red, QiKO. Haems b (peaks at 434 and 564 nm) and ci (broad band at 425 nm) components36; in QiKO the decreased amplitude at 434 and 564 nm demonstrates the absence of bh and the trough at 420 nm the absence of ci. The spectra have been normalized to the cytochrome f content (inset).