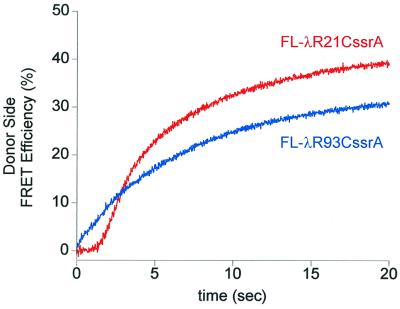
Figure 4.
FRET between donor-labeled EDANS-miClpP and acceptor-labeled Fl-λRssrA initiates earlier during a ClpA-mediated translocation reaction for a substrate probe that is adjacent to the COOH-terminal ssrA tag. ClpA (2 μM hexamer), miClpP (2 μM tetradecamer), either Fl-λRssrA (2 μM), and ATPγS (1 mM) were incubated together in reaction buffer at 25°C for 45 min, then rapidly mixed with an excess of ATP (10 mM) in the stopped-flow apparatus. Each trace is the sum of four runs. The difference in the apparent rate of acquisition of FRET between the two Fl-λRssrA molecules may reflect an early step in the overall reaction that is largely complete during the lag phase for the NH2-terminally labeled substrate, but that contributes to the apparent rate of the COOH-terminal one. The difference in the final FRET efficiency reached with the two molecules (42% vs. 34%, respectively) likely results from asymmetric binding of the tagged substrate within the ClpP chamber and, hence, different distances and relative orientations between the probes.