Abstract
Background
Esophagitis caused by gastroesophageal reflux disease (GERD) results in appreciable morbidity and economic burden. No systematic review has addressed the effectiveness of prokinetic drugs in the treatment of GERD esophagitis in adults.
Objective
To determine the utility of prokinetic drugs in improving symptoms and endoscopic lesions in patients with GERD esophagitis.
Methods
We included randomized controlled trials that compared prokinetic drugs with placebo. A systematic search included the Cochrane Controlled Trial Register, MEDLINE, CINAHL, LILACS, EMBASE, a manual search of books and article references, and contact with pharmaceutical companies. Reviewers assessed methodological quality and extracted data that were combined using a random effects model.
Results
Eighteen articles met the eligibility criteria; of these, 13 used prokinetic drugs alone, 4 tested prokinetic drugs as additional therapy in patients receiving histamine-2 receptor blockers, and 1 tested them in patients receiving proton pump inhibitors. Seven studies evaluated clinical improvement only, 5 addressed endoscopic improvement only, and 6 reported both outcomes. Four studies failed to provide adequate data for pooling; 3 of the 4 reported results that suggested symptomatic benefit with prokinetic agents. Nine studies (379 patients) that provided the required data suggested a higher incidence of clinical improvement with prokinetic drugs versus placebo (relative risk [RR] 1.70, 95% confidence interval [CI] 1.37–2.12, heterogeneity p = 0.47, I2 = 0%). Clinical improvement occurred in 53 out of 175 patients (30%) of the control group; applying the relative risk of 1.70 and associated confidence interval suggests that absolute increases in patients improved might vary from 18% to 41% (number needed to treat approximately 3 to 6). Improvement was similar in 4 studies in which the prokinetic agent was added to an antisecretory drug. The funnel plot, however, suggests the possibility of publication bias. Eleven studies (887 patients) suggested a higher likelihood of endoscopic improvement or healing esophagitis with prokinetic drugs (RR 1.26, 95% CI 1.03–1.53) but with significant heterogeneity (heterogeneity p = .05, I2 = 46.2%) that we couldn’t explain with an a priori hypothesis. When we evaluated endoscopic healing as the main outcome we observed a trend toward better results in the treatment group, also with inexplicable heterogeneity (RR 1.36, CI 95% 0.97–1.89, I2 = 61%).
Conclusions
Randomized controlled trials provide moderate-quality evidence that prokinetic drugs improve symptoms in patients with reflux esophagitis and low-quality evidence that they have an impact on endoscopic healing.
Esophagitis is a frequent complication of gastroesophageal reflux disease (GERD). The diversity of clinical manifestations and the lack of standardized diagnostic criteria across studies create difficulties in estimating its prevalence.1, 2 Pathophysiologic mechanisms include anatomic and functional changes of the gastroesophageal junction (hiatal hernia, decrease of the inferior esophageal sphincter tone and esophageal clearance).3 Definitive diagnosis of esophagitis requires endoscopy and biopsy.4
Chronic esophagitis complications include bleeding, esophageal stenosis, Barrett metaplasia and adenocarcinoma. The goal of medical treatment is to decrease symptoms and complications by the suppression of gastric acid secretion and by ameliorating motor dysfunction. Therapeutic options include proton pump inhibitors (PPIs), histamine-2 receptor (H2) antagonists and prokinetic drugs.
Prokinetic drugs have potential usefulness as adjunctive treatment of GERD by increasing lower esophageal sphincter pressure, enhancing gastric emptying, and improving peristalsis. A clinical practice guideline on GERD esophagitis1 suggested the potential benefit of promotility agents, either as monotherapy or used in association with PPI. The authors emphasized the need for continued research into the role of these agents.
Any further research or recommendations regarding prokinetic agents should, however, be based on a systematic summary of evidence to date. Although systematic reviews have examined the short-term impact of prokinetic agents5 on gastroesophageal reflux symptoms in patients without endoscopically proven esophagitis,6 no systematic review has evaluated their effect on endoscopically proven esophagitis in adults. We therefore undertook a systematic review and meta-analysis to evaluate the real effectiveness of prokinetic drugs in patients with proven GERD esophagitis.
Methods
Eligibility criteria
We included all published and unpublished parallel-group randomized or quasi-randomized controlled trials published in Spanish, English, French, German, Italian or Portuguese that met the following criteria:
Patients: adults > 15 years with endoscopic diagnosis of reflux esophagitis (with or without histology).
Intervention: use of oral prokinetic agents (cisapride, mosapride, tegaserod, metoclopramide, domperidone, bethanechol, levosulpiride, cinitapride, clebopride) compared with placebo. Studies in which patients received antisecretory agents (PPI or H2 antagonists) were included only if both the treatment and control groups received these agents according to the same protocol.
Outcomes: symptomatic improvement (heartburn, regurgitation, dysphagia, retrosternal pain) or endoscopic findings.
We excluded studies with the following characteristics:
Patients: those with esophageal involvement of a systemic illness (scleroderma, dermatomyositis), dysphagia of neurologic cause, previous gastrectomy or antireflux surgery.
Intervention: use of prokinetics after satisfactory treatment with PPI or for symptomatic relapse.
Trial design: trials with scores of ≤ 3 in the Jadad scale modified by Schulz criteria (score 0 to 8).7
Titles and abstracts were independently reviewed by 2 of the authors (MEM and FAS) to identify potentially eligible articles. We obtained full-text versions of potentially eligible articles, which the same 2 reviewers evaluated. In case of disagreement, 1 of 3 other reviewers (MFK, GG and HNC) made the eligibility decision.
Search strategy
We searched relevant articles in the following electronic databases: LILACS (1985–2007), MEDLINE (1966–2007), EMBASE (1980–2007), CINAHL (1982–2007), COCHRANE Controlled Trial Register (Cochrane Library 2007). The terms used were the names of prokinetic agents (both generic and proprietary), combined with reflux esophagitis and therapeutic categories. We also hand-searched abstracts reported at the 7th United European Gastroenterology Week (UEGW, November 1999), the 11th UEGW (November 2003) and at the Digestive Diesase Week and the 104th Annual Meeting of the American Gastroenterological Association (May 2003) We reviewed the reference lists of included articles, other sources such as UptoDate (2007 version 15.1) and relevant gastroenterology, pharmacology and internal medicine textbooks. We also contacted a local expert and 5 pharmaceutical companies (Beta, Roux-Ocefa, Janssen-Cilag, Phoenix, and Cetus) to identify unpublished articles.
Quality
Two of the authors (MEM and FAS) independently evaluated concealment allocation, blinding and completeness of follow-up. The reviewers used the Jadad scale modified by Schulz criteria to evaluate and classify the quality of the studies.7
Data abstraction
Two of the authors (MEM and FAS) independently abstracted the data in duplicate. Patient characteristics, interventions (drugs used, dose, time of administration, co-interventions), outcome measures (symptomatic or endoscopic response, endoscopic healing and adverse events) were abstracted, and any disagreement was resolved through discussion. We made attempts to contact authors regarding confirmation or missing data; 2 authors answered our request. Abstracts provided no eligible studies.
Quantitative data synthesis and statistical analysis
We used weighted kappa scores to assess agreement between the reviewers on the selection of articles for inclusion. We calculated relative risk (RR) and absolute risk reduction (ARR) with 95% confidence intervals (CIs) for symptomatic and endoscopic response, and combined the RR from each study by means of a meta-analytic technique using a random effects model as described by DerSimonian and Laird.8 RevMan 4.2 was used to analyze all data.
The authors of the trials used different scores to assess improvement (e.g., 0 to 100 symptom scale; categorical scale from absent to disabling symptoms). We used the authors’ own criteria in each trial to classify patients as improved or unimproved. We considered outcomes of patients free of symptoms and patients with symptomatic improvement as equivalent, and pooled each outcome of interest based on the a priori expectation of a similar magnitude and direction of treatment effect. We classified “some improvement” with complete symptom resolution as a positive outcome and “no improvement” as a negative outcome.
Heterogeneity of the studies was evaluated both by the chi-square test with a threshold p value of < 0.05, and by the I2 statistic (considering important heterogeneity a proportion higher than 30%). For any outcome that crossed either threshold for heterogeneity, we explored sources of heterogeneity according to our a priori hypothesis, which included drugs used, the use of additional agents, dosing, duration of treatment and methodological quality. Specifically, we compared the results of studies grouped by the following factors:
different drugs used (cisapride, metoclopramide, bethanechol, levosulpiride, domperidone, mosaprid) use of antisecretory agents (yes or no)
dose of prokinetic drugs used per day (< 40 mg v. ≥ 40 mg of cisapride, < 40 mg v. ≥ 40 mg metoclopramide)
treatment duration (≤ 8 weeks v. > 8 weeks)
methodological qualtity (Jadad score 4 v. ≥ 5).
We used log-transformed RR and its standard error calculated from 95% CI and Z value to obtain p values for testing explanations of heterogeneity.
All data were analyzed on an intention-to-treat principle. Publication bias was evaluated using funnel plots.
Results
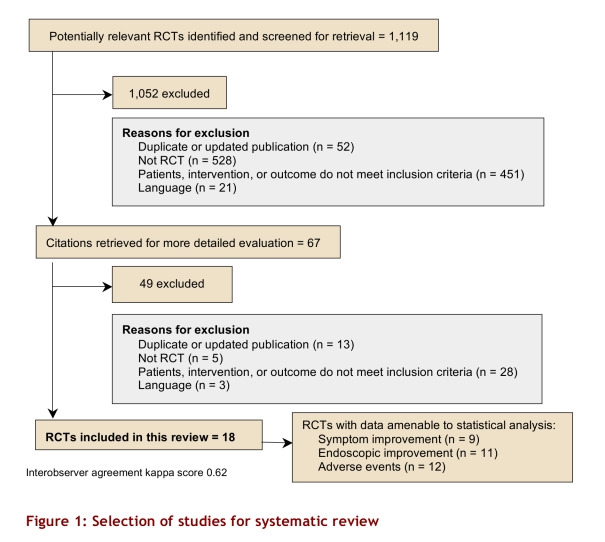
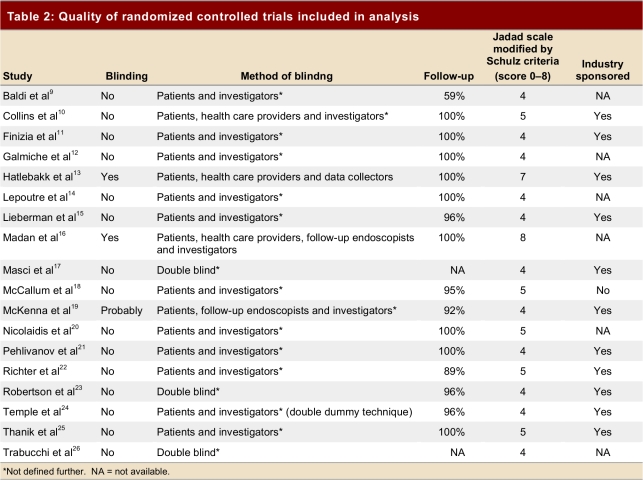
We identified 1,119 abstracts (Figure 1). None of 15 studies provided by pharmaceutical companies fulfilled eligibility criteria. Eighteen9-26 randomized controlled trials (RCTs) enrolling 1,155 patients (621 in the intervention and 504 in the placebo group; Table 1), all identified from electronic databases, proved eligible. Table 2 shows characteristics and methodological quality of the eligible trials. Eleven trials were rated 4 using the Jadad scale modified by Schulz criteria, and 7 were rated 5 or more; 11 trials were sponsored by pharmaceutical industry. Six RCTs failed to provide sponsorship information, and 1 had no sponsor.
Figure 1.
Selection of studies for systematic review
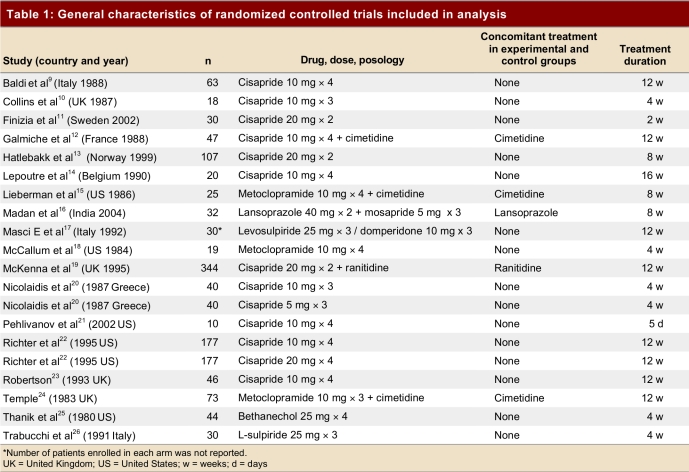
Table 1.
General characteristics of randomized controlled trials included in analysis
Table 2.
Quality of randomized controlled trials included in analysis
Eleven trials evaluated the effect of cisapride. Of these, 1 trial evaluated 80 mg/d dose, 9 trials the 40 mg/d dose, 2 trials the 30 mg/d dose and only 1 trial the 15 mg/d dose. Metoclopramide was evaluated in 3 studies; 1 evaluated the 30 mg/d dose and the other 2 the 40 mg/d dose. One study evaluated bethanechol, 1 study evaluated mosapride and 2 studies evaluated each of sulpiride and domperidone. Four trials compared the addition of a prokinetic drug versus placebo in patients already using an H2 receptor blocker (3 cimetidine and 1 ranitidine). One trial evaluated the addition of a prokinetic agent to a PPI.
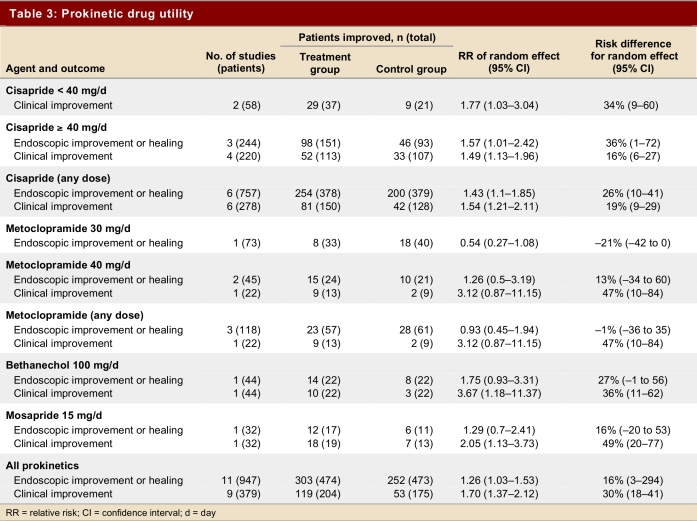
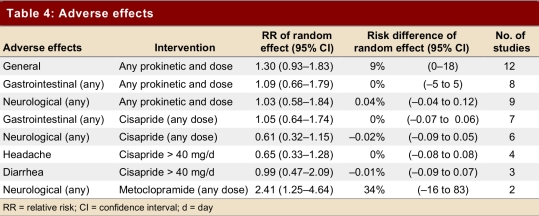
Table 3 and Table 4 show primary outcomes and adverse reactions.
Table 3.
Prokinetic drug utility
Table 4.
Adverse effects
Studies that did not provide data for pooling
Masci and colleagues17 evaluated the presence and severity of reflux symptoms (dysphagia, regurgitation, heartburn, retrosternal pain, nausea), comparing levosulpiride, domperidone and placebo. They reported equal effectiveness of both drugs in significantly reducing regurgitation, heartburn and overall dyspeptic symptoms (p < 0.05 compared with control). Endoscopic features failed to reveal significant differences between groups.
Trabucchi and colleagues26 compared levosulpiride with placebo. They reported improvement in symptom score in most patients in the treatment group. Endoscopic lesions disappeared in 20%, improved in 47% and failed to improve in 33%. The authors did not provide data for the placebo arm.
Finizia and colleagues11 reported no significant difference on symptom score according to intensity, frequency and duration in the cisapride group compared to placebo.
Pehlivanov and colleagues21 found fewer heartburn episodes during daytime and fewer antacid tablets needed per patient in a week in the cisapride group versus the placebo group, with a p value of 0.016 and 0.062, respectively.
Clinical improvement
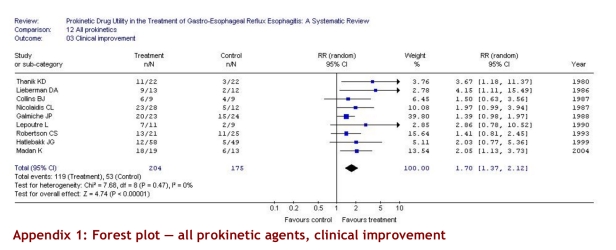
Nine studies (379 patients) evaluated clinical improvement. The pooled estimate showed a significant improvement with prokinetic drugs versus placebo (RR 1.70, 95% CI 1.37–2.12). Results were consistent across studies (p = 0.47, I2 = 0%). The ARR was 30% (95% CI 18%–41%) (Table 3).
Endoscopic improvement
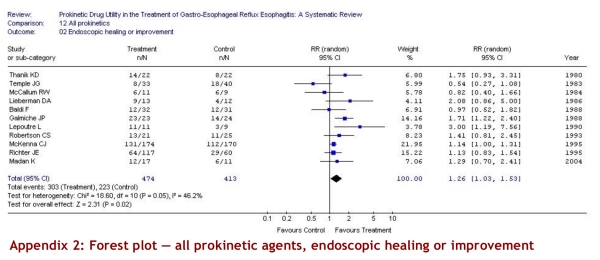
Five of 11 studies used a validated scale (Savary-Miller [3], Los Angeles [1], Hetzel [1]); the other 6 scales used were similar to one another. The pooled estimate of treatment effect on endoscopic healing or improvement from 11 studies including 887 patients demonstrated a significant effect of prokinetic drug versus placebo (RR 1.26, 95% CI 1.03–1.53); the risk difference was 16% (CI 95% 3%–29%). The results were, however, variable from study to study (test for heterogeneity p = 0.05, I2 = 46.2%). We therefore explored the possible sources of heterogeneity according to our a priori hypothesis. The a priori hypotheses failed to explain the variability in study results.
Endoscopic healing
The pooled estimate of treatment effect on endoscopic healing from eight studies including 796 patients demonstrated a trend toward better results with prokinetic treatment (RR 1.36, 95% CI 0.97–1.89) with significant heterogeneity between groups (test for heterogeneity p = 0.001, I2 = 61.4%). Once again, our a priori hypotheses failed to explain the variability observed.
Adverse events
Twelve trials reported adverse events. They showed a non-significant increase in adverse reactions (RR 1.30, 95% CI 0.93–1.83), with substantial variability between the studies (test for heterogeneity p = 0.08, I2 = 39.4%); the risk difference was 9% (CI 95% 0%–18%) (Table 4).
Discussion
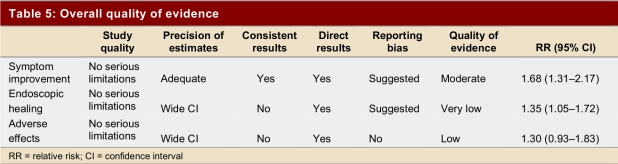
Our intention was to determine the quality of evidence and apparent magnitude of impact of prokinetic agents on symptoms, endoscopic healing, and adverse effects in patients with gastroesophageal reflux. We found an increase in the probability of symptom improvement of 70% with treatment (RR 1.70, 95% CI 1.37–2.12), and an increase of 26% (RR 1.35, 95% CI 1.03–1.53) in the probability of endoscopic healing or improvement, but we did not find a significant increase in adverse reactions with treatment (RR 1.30, 95% CI 0.93–1.83). The last 2 outcomes showed substantial variability between the studies.
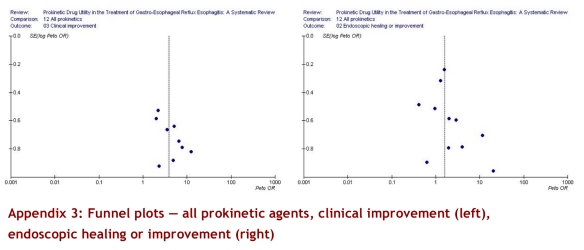
The GRADE system of rating quality of evidence provides a structure for assessing the quality of the evidence.27 In the GRADE system, randomized trials constitute high-quality evidence unless there are important limitations. The 12 trials that addressed symptomatic improvement were of moderate to high quality; their results were consistent, the confidence intervals reasonably narrow, and the results applied directly to the relevant population (Table 5). However, the trials were small, a number were funded by industry, and the funnel plot (see Appendices) suggests the possibility of publication bias — a substantial risk when evidence comes from a number of small trials. In our judgment, therefore, we have moderate evidence of symptomatic benefit with prokinetic agents.
Table 5.
Overall quality of evidence
With respect to endoscopic improvement, the 10 relevant randomized trials were of moderate to high quality and provide direct evidence regarding the impact of prokentic agents. The results, however, were not consistent across studies (test for heterogeneity p = 0.05, I2 = 46.2%) and our a priori hypotheses failed to explain differences in the magnitude of effect across studies. Moreover, the confidence intervals around the effect were wide (RR 1.26, 95% CI 1.03–1.53), and the pattern of results suggest the possibility of publication bias. We therefore conclude that the results provide only weak evidence supporting the benefits of prokinetic agents on endoscopic healing.
With respect to adverse effects, these randomized trials were of moderate to high quality and the results are directly applicable to the patient population. The results are, however, inconsistent (test for heterogeneity p = 0.08, I2 = 39.4%) and the confidence intervals wide (RR 1.30, 95% CI 0.93–1.83). The results therefore provide weak evidence of toxicity of prokinetic agents. Furthermore, the studies are extremely underpowered to detect rare but serious side effects. Case reports of cardiovascular adverse effects remain, therefore, an important concern.28-30
Strengths of our systematic review include explicit, detailed eligibility criteria; a comprehensive search; restriction to RCTs of moderate or high methodological quality; high levels of agreement on issues requiring judgment; and our use of the systematic GRADE approach to rate the quality of the evidence. Limitations pertain to the number of patients studied and the methodological concerns that we have highlighted (in particular, the possibility of publication bias). With respect to relevance, although cisapride is no longer available in most markets, an alternative agent, mosapride, may take its place. The one trial of mosapride in this review is notable both for its high methodological quality and the fact that it detected a benefit of mosapride in patients already receiving a PPI.
Although the enthusiasm for PPIs in the treatment of reflux esophagitis is appropriate, we do not believe it warrants the neglect of other agents. The previous lack of systematic review and meta-analysis, as presented here reflects, in our view, a certain neglect.
In summary, although patients with reflux esophagitis are likely to benefit symptomatically from the use of prokinetic agents, some uncertainty remains. The magnitude of side effects and toxicity is uncertain. Clinicians must also consider the higher-quality evidence available for other agents (particularly PPIs, which are superior to prokinetic agents) in making their therapeutic decisions.
Biographies
Matías E Manzotti, Fernando A Serrano, Gisela Di Stilio and María F Koch are attending physicians in general internal medicine, and
Hugo N Catalano is clinical director of general internal medicine in the Department of Internal Medicine, Hospital Alemán de Buenos Aires, School of Medicine, Buenos Aires University, Buenos Aires, Argentina
Gordon H Guyatt is a professor in the Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Ont., Canada.
Appendix
Appendix 1.
Forest plot — all prokinetic agents, clinical improvement
Appendix 2.
Forest plot — all prokinetic agents, endoscopic healing or improvement
Appendix 3.
Funnel plots — all prokinetic agents, clinical improvement (left), endoscopic healing or improvement (right)
Footnotes
Competing interests: None declared.
Funding source: None.
References
- 1.DeVault Kenneth R, Castell Donald O. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100(1):190–200. doi: 10.1111/j.1572-0241.2005.41217.x. http://www.nature.com/doifinder/10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 2.Dent J, El-Serag H B, Wallander M-A, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–717. doi: 10.1136/gut.2004.051821. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/15831922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunnett NW, Lingappa VR. Reflux esophagitis. In: McPhee Stephen J, Lingappa Vishwanath, Ganong William., editors. Pathophysiology of disease: an introduction to clinical medicine. 5th ed. New York: McGraw-Hill; 2006. [Google Scholar]
- 4.Peters J H, DeMeester T R. Esophagus and diaphragmatic hernia. Part II: specific considerations. In: Brunicardi F, Andersen Dana, Billiar Timothy, Dunn David, Hunter John, Pollock Raphael E, editors. Schwartz's Principles of Surgery. 8th ed. New York: McGraw-Hill; 2005. [Google Scholar]
- 5.van Pinxteren B, Numans M E, Bonis P A, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD002095.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Augood C, MacLennan S, Gilbert R, Logan S. Cisapride treatment for gastro-oesophageal reflux in children. Cochrane Database Syst Rev. 2003;(4) doi: 10.1002/14651858.CD002300. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Cook D J, Jadad A R, Tugwell P, Moher M, Jones A, Pham B, Klassen T P. Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess. 1999;3(12) http://www.hta.ac.uk/execsumm/summ312.htm. [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Baldi F. Cisapride versus placebo in reflux esophagitis: a multicenter double-blind trial. J Clin Gastroenterol. 1988;10(6):614–618. doi: 10.1097/00004836-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Collins B J, Spence R A, Ferguson R, Laird J, Love AH. Cisapride: influence on oesophageal and gastric emptying and gastro-oesophageal reflux in patients with reflux oesophagitis. Hepatogastroenterology. 1987;34(3):113–116. doi: 10.1097/00004836-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Finizia Caterina, Lundell Lars, Cange Lars, Ruth Magnus. The effect of cisapride on oesophageal motility and lower sphincter function in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2002;14(1):9–14. doi: 10.1097/00042737-200201000-00003. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00042737-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Galmiche J P, Brandstätter G, Evreux M, Hentschel E, Kerstan E, Kratochvil P, Reichel W, Schütze K, Soule J C, Vitaux J. Combined therapy with cisapride and cimetidine in severe reflux oesophagitis: a double blind controlled trial. Gut. 1988 May 1;29(5):675–681. doi: 10.1136/gut.29.5.675. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/3294124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatlebakk J G, Hyggen A, Madsen P H, Walle P O, Schulz T, Mowinckel P, Bernklev T, Berstad A. Heartburn treatment in primary care: randomised, double blind study for 8 weeks. BMJ. 1999 Aug 28;319(7209):550–553. doi: 10.1136/bmj.319.7209.550. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/10463897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepoutre L, Van der Spek P, Vanderlinden I, Bollen J, Laukens P. Healing of grade-II and III oesophagitis through motility stimulation with cisapride. Digestion. 1990;45(2):109–114. doi: 10.1159/000200231. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman D A, Keeffe E B. Treatment of severe reflux esophagitis with cimetidine and metoclopramide. Ann Intern Med. 1986;104(1):21–26. doi: 10.7326/0003-4819-104-1-21. [DOI] [PubMed] [Google Scholar]
- 16.Madan K, Ahuja V, Kashyap P C, Sharma M P. Comparison of efficacy of pantoprazole alone versus pantoprazole plus mosapride in therapy of gastroesophageal reflux disease: A randomized trial. Dis Esophagus. 2004;17(4):274–278. doi: 10.1111/j.1442-2050.2004.00424.x. [DOI] [PubMed] [Google Scholar]
- 17.Masci E, Sorghi M, Tosi T, Testoni P A, Tottobello A. Levosulpiride and domperidone in the treatment of reflux esophagitis: results of a double-blind study versus placebo. Curr Ther Res Clin Exp. 1992;51(6):814–818. [Google Scholar]
- 18.McCallum R W, Fink S M, Winnan G R, Avella J, Callachan C. Metoclopramide in gastroesophageal reflux disease: rationale for its use and results of a double-blind trial. Am J Gastroenterol. 1984;79(3):165–172. [PubMed] [Google Scholar]
- 19.McKenna C J, Mills J G, Goodwin C, Wood J R. Combination of ranitidine and cisapride in the treatment of reflux oesophagitis. Eur J Gastroenterol Hepatol. 1995;7(9):817–822. [PubMed] [Google Scholar]
- 20.Nicolaidis C L, Kehagiolou K, Mantzanaris G, Papadatou-Marinou A, Saklaridis J, Georgiotis D, et al. Therapeutic effect of two dosages of cisapride in controlling chronic reflux symptoms in esophagitis patients. Curr Ther Res Clin Exp. 1987;42(6):1059–1065. [Google Scholar]
- 21.Pehlivanov N, Sarosiek I, Whitman R, Olyaee M, McCallum R. Effect of cisapride on nocturnal transient lower oesophageal sphincter relaxations and nocturnal gastro-oesophageal reflux in patients with oesophagitis: a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2002;16(4):743–747. doi: 10.1046/j.1365-2036.2002.01225.x. http://doi.wiley.com/10.1046/j.1365-2036.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 22.Richter J E, Long J F. Cisapride for gastroesophageal reflux disease: a placebo-controlled, double-blind study. Am J Gastroenterol. 1995;90(3):423–430. [PubMed] [Google Scholar]
- 23.Robertson C S, Evans D F, Ledingham S J, Atkinson M. Cisapride in the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1993;7(2):181–190. doi: 10.1111/j.1365-2036.1993.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 24.Temple J G, Bradby G V, O'Connor F O, Panesar K S, Mulligan T O, Robinson T J, Ward D W. Cimetidine and metoclopramide in oesophageal reflux disease. Br Med J (Clin Res Ed) 1983 Jun 11;286(6381):1863–1864. doi: 10.1136/bmj.286.6381.1863. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/6407606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thanik K D, Chey W Y, Shah A N, Gutierrez J G. Reflux esophagitis: effect of oral bethanechol on symptoms and endoscopic findings. Ann Intern Med. 1980;93(6):805–808. doi: 10.7326/0003-4819-93-6-805. [DOI] [PubMed] [Google Scholar]
- 26.Trabucchi E, Radaelli E, Castoldi L, Abelli P, Brambilla A, Fichera G, Diamantini S, Pace M, Foschi D. The effects of L-sulpiride on reflux oesophagitis. Drugs Exp Clin Res. 1991;17(6):317–321. [PubMed] [Google Scholar]
- 27.Guyatt G, Gutterman D, Baumann M H, Addrizzo-Harris D, Hylek E M, Phillips B. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174–181. doi: 10.1378/chest.129.1.174. http://www.chestjournal.org/cgi/pmidlookup?view=long&pmid=16424429. [DOI] [PubMed] [Google Scholar]
- 28.Wysowski D K, Bacsanyi J. Cisapride and fatal arrhythmia. N Engl J Med. 1996 Jul 25;335(4):290–291. doi: 10.1056/NEJM199607253350416. [DOI] [PubMed] [Google Scholar]
- 29.Piquette R K. Torsade de pointes induced by cisapride/clarithromycin interaction. Ann Pharmacother. 1999;33(1):22–26. doi: 10.1345/aph.18107. [DOI] [PubMed] [Google Scholar]
- 30.Vitola J, Vukamovic J, Roden DM. Cisapride-induced torsades de pointes. J Cardiovasc Electrophysiol. 1998;9(10):1109–1113. doi: 10.1111/j.1540-8167.1998.tb00888.x. [DOI] [PubMed] [Google Scholar]