Figure 6.
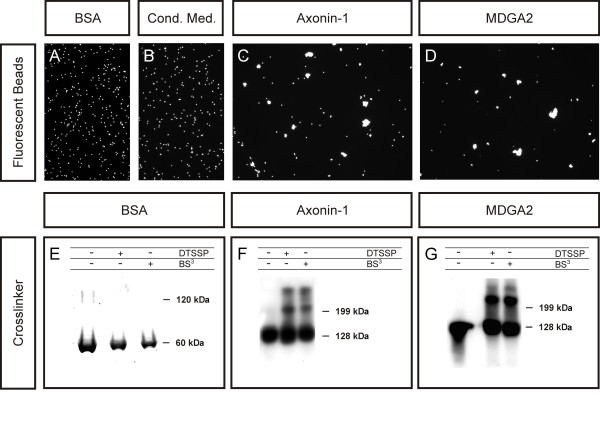
MDGA2 is capable of interacting homophilically. Two independent assays were used to analyze the binding capabilities of MDGA2. In an aggregation assay, fluorescent beads were coupled with BSA, proteins from conditioned medium of non-transfected cells, recombinant axonin-1 or recombinant MDGA2 and analyzed for their aggregation behaviour. (A-D) While no aggregates were observed with BSA (A) or beads coated with proteins released by mock-transfected cells (B), strong aggregation was detected with axonin-1- (C) and MDGA2-coupled beads (D). (E-G) Similar results were seen when chemical cross-linkers were used to monitor the binding capabilities of these molecules. (E) Silver staining of gels indicated that, in a concentrated BSA solution, no high molecular weight aggregates were formed in the presence of the chemical cross-linkers. Crosslinking of conditioned media containing recombinant flag-tagged axonin-1 (F) or flag-tagged MDGA2 (G) resulted in a molecular weight shift of a substantial part of the detected protein as observed by western blot assays using anti-flag antibodies. In the case of axonin-1, note that two additional bands are observed in the presence of chemical cross-linkers, indicating that axonin-1 is capable of forming multimeric complexes.