Abstract
Objectives:
To evaluate the antioxidant and free-radical scavenging activities of triethylchebulate (TCL), an aglycone isolated from the fruit of Terminalia chebula Retz.
Materials and Methods:
Microsomes, mitochondria and red blood cells (RBCs) were isolated from rat liver. The antioxidant capacities were evaluated by determining the inhibitory effects of TCL on lipid peroxidation, hydrogen peroxide (H2O2)-induced RBCs hemolysis and RBCs autoxidative hemolysis. The free-radical scavenging activities were evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) method and 2´,7´-dichlorodihydrofluorescin diacetate (DCFH2-DA) assay.
Result:
TCL significantly inhibited FeSO4/Cys-induced microsomes lipid peroxidation and protected both H2O2--induced RBCs hemolysis and RBCs auto-hemolysis in a dose-dependent manner. Furthermore, TCL demonstrated potent DPPH free-radical scavenging ability with IC50 at 2.4×10-5 M. In addition, TCL also moderately suppressed azide-induced mitochondria ROS formation.
Conclusion:
These results demonstrated that TCL was a strong antioxidant and free-radical scavenger, which might contribute to the anti-oxidative ability of Terminalia chebula Retz.
Keywords: Anti-oxidant, hemolysis, lipid peroxidation, reactive oxygen species, Terminalia chebula Retz, triethylchebulate
Introduction
Terminalia chebula Retz is a kind of large tree distributed throughout the world. The fruits and barks of Terminalia chebula Retz had been used as an ethnodrug from ancient times. In Myanmar, medicinal terminalia fruit is used as a laxative and a tonic. In China, it is applied as a carminative, astringent and texpectorant, and also as a remedy for excessive salivation and heartburn. In Indo-China, it is regarded as a purgative agent. In Malaysia, medicinal terminalia fruit is believed to exhibit anti-diarrheic, styptic, anti-bilious and anti-dysenteric activities.[1] Previous studies revealed that Terminalia chebula Retz extract showed anti-bacterial,[2,3] anti-proliferative[4] and anti-diabetic activities.[5] Chebulagic acid isolated from the fruits was identified as a COX-2 and LOX-5 dual inhibitor and showed anti-proliferative and pro-apoptotic activities in several tumor cell lines.[6] Recently, Terminalia chebula Retz extract was reported to possess against blood-sucking parasites[7] and cytochrome P450 inhibition effect.[8] Our previous study found that arjunic acid, a triterpene isolated from Terminalia chebula Retz is a potent-free radical scavenger.[9] Triethylchebulate (TCL) is an aglycone isolated from Terminalia chebula Retz [Figure 1].[10] However, little is known about its bioactivity and pharmacological effects. In this study, its antioxidant capacity was evaluated extensively in vitro.
Figure 1.
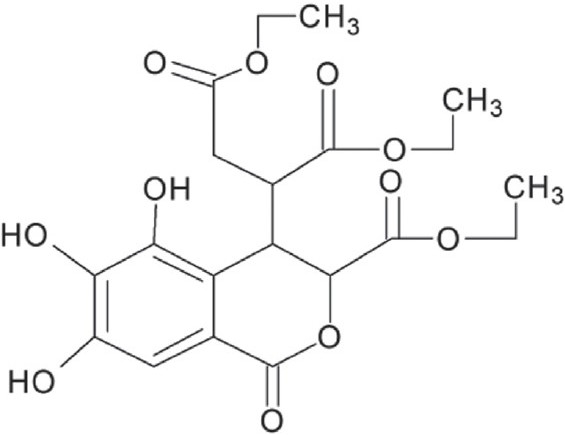
Chemical structure of triethylchebulate
Materials and Methods
Reagents and Animals
TCL (>95%) was isolated and identified according to our previous report.[11] Dry fruit of Terminalia chebula Retz (9 kg) was extracted with 95% ethanol, petroleum ether, ethyl acetate and 5% NaHCO3 sequentially, each for three times. The obtained extract (150 g) was isolated with a silica gel column chromatograph using chloroform/methanol gradient. TCL (900 mg) was collected at the gradient (50: l). Cysteine, trichloroacetic acid (TCA), thiobarbituric acid (TBA), butylated hydroxytoluene (BHT), ascorbic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferrous sulfate (FeSO4), 2´,7´-dichlorodihydro-fluorescindiacetate (DCFH2-DA) were purchased from Sigma (USA).
The male Wistar rats (200–250 g) were purchased from Experimental Animal Center of Chinese Academy of Medical Sciences. All studies were approved by the Laboratories Institutional Animal Care and Use Committee of Chinese Academy of Medical Sciences and Peking Union Medical College.
Preparation of Microsomes, Red Blood Cells and Mitochondria
Rats were starved overnight before experiment and the liver microsomes were isolated as previously reported.[12] Protein content was determined by Lowry assay.[13]
Red blood cells (RBCs) were isolated from blood by centrifugation and gently suspended with PBS-glucose (10 mM) to obtain proper percentage hematocrit.[14]
Liver mitochondria were isolated as previous described and resuspended in determination medium.[15] The protein content was determined by Lowry assay.[13]
Effect on Lipid Peroxidation
The effect of TCL on lipid peroxidation was performed by the measurement of TBARS.[16] In brief, microsomes were incubated at 37°C in 0.1 M PBS, pH 7.5, and made up to a final protein concentration of 0.5 mg/ml. The peroxidation was initiated by 0.01 M cysteine and 1 mM ferrous sulfate (FeSO4) (final concentration) in a total volume of 1.0 ml. The reaction mixture was gently shaken at 37°C for 30 min, followed by addition of a TCA-TBA-HCl stock solution (15% w/v trichloroacetic acid; 0.375% w/v TBA; 0.25 N HCl) together with 0.02% w/v BHT. This amount of BHT completely prevents the formation of any non-specific TBARS.[17] The solution was heated in a boiling water bath for 15 min. After centrifugation, the TBARS in the supernatant was determined at 532 nm[18] with SPECTRA-max M5 (Molecular Devices, USA). Ascorbic acid was used as a reference compound.
Effect on H2O2-induced RBCs Hemolysis
Freshly prepared RBCs were gently resuspended with PBS-glucose to obtain a 5% hematocrit, and pre-incubated at 37°C for 10 min in the presence of 1 mM sodium azide (to inhibit catalase activity). Subsequently, they were divided into various aliquots of 1 ml for each experimental treatment. All of these, except the control tubes, were challenged with 10 mM H2O2 with or without the addition of different concentrations of TCL. After 60 min at 37°C, cells were kept for 60 s in an ice bath and then centrifuged at 1800 g for 10 min at 4°C. About 200 μl of the supernatant was taken to a 96-well plate and the absorbance was read at 514 nm[19] with SPECTRA-max M5 (Molecular Devices, USA). Ascorbic acid was used as reference compound.
Effect on RBCs Autoxidative Hemolysis
One milliliter of 1% hematocrit RBCs was taken to a test tube and different concentrations of TCL were added. The mixture was incubated in a water bath at 37°C. The test tubes were oscillated gently every 2 h, and after 24 h of incubation the test tubes were then centrifuged at 1800 g for 10 min. Two hundred microliters of the supernatant was taken to a 96-well plate and the absorbance was read at 540 nm[20] SPECTRA-max M5 (Molecular Devices, USA). Ascorbic acid was used as reference compound.
DPPH Free Radical Scavenging Activity
The DPPH radical-scavenging effect was measured according to the method of Kang and Saltveit.[21] Different concentrations of TCL (10 μl) were mixed with 190 μl DPPH radical (1 μM) in a methanol solution. After 20-min incubation at room temperature in the dark, the absorbance was read at 517 nm[22] with SPECTRA-max M5 (Molecular Devices, USA). Ascorbic acid was used as reference compound.
Effect on Sodium Azide Induced Mitochondrial ROS Production
The DCFH2-DA assay was performed in a volume of 200 μl reaction system in black 96-well plates containing determination medium (final concentration) (NaCl 153 mM, KCl 2.68 mM, MgCl2 1.0 mM, Tris-HCl 10 mM, glucose 20 mM, pH 7.4), 0.3 mg/ml (mitochondrial protein), 1 mM sodium succinate, 2 μM DCFH2-DA and 0.1 mM sodium azide. TCL was added to the mitochondria solution before the addition of sodium azide to minimize DCFH2-DA oxidation by the radicals produced with sodium azide and the sodium succinate was added at last to initiate mitochondria respiration.[23] After a few seconds of oscillation, the plates were kept at 37°C for 30 min in the dark and the fluorescence was determined at 492 nm/520 nm[24] with SPECTRA-max M5 (Molecular Devices, USA). Ascorbic acid was used as reference compound.
Statistical Analysis
Data are expressed as mean ± SD. The significance of intergroup differences was evaluated by one-way analyses of variance (one-way-ANOVA) using software SPSS11.5. Differences were considered significant at P < 0.05.
Results
In the FeSO4/Cys-induced microsomes lipid peroxidation model, TCL has a strong inhibitory effect on FeSO4/Cys-induced MDA formation as determined by TBARS assay. Furthermore, a dose-dependent inhibitory effect was observed. Compared with the same concentration of ascorbic acid, TCL showed a much higher inhibitory effect [Figure 2].
Figure 2.
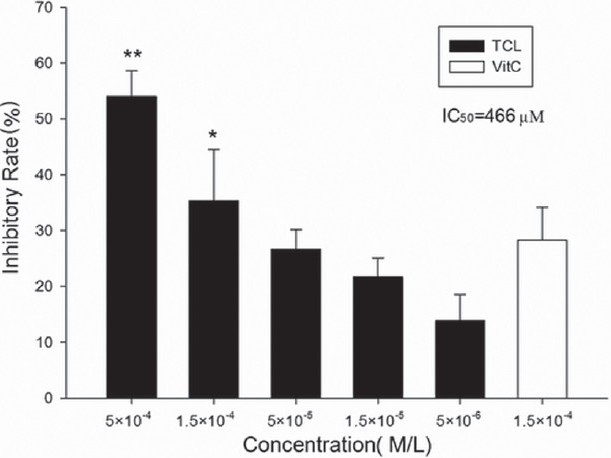
Inhibitory effect of TCL on rat liver microsomal MDA formation (∗∗P<0.01 versus ascorbic acid, ∗P<0.05 versus ascorbic acid). Vit. C, ascorbic acid
TCL significantly decreased H2O2-induced RBCs hemolysis in a dose-dependent manner and even at as low as 5×10-6 M it showed clear inhibitory effect (25.5%±4.6%). The calculated IC50 was 4.2×10-5 M. Compared with the same concentration of ascorbic acid, TCL showed a much higher inhibition [Figure 3]. Similar inhibitory effect on RBCs autoxidative hemolysis was also observed but with much lower inhibitory rate [Figure 4].
Figure 3.
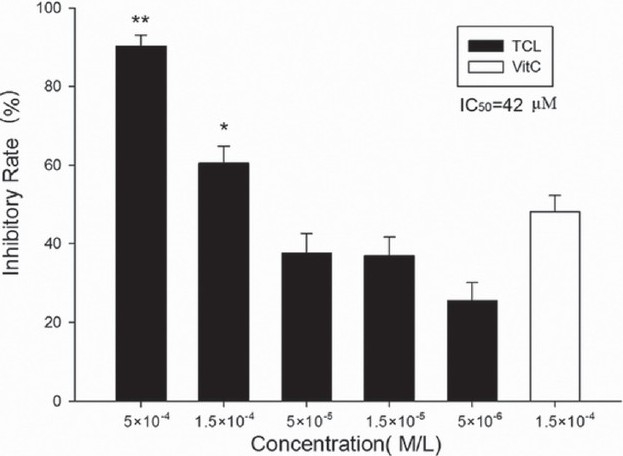
Inhibitory effect of TCL on H2O2-induced RBCs hemolysis (∗∗P<0.01 versus ascorbic acid, ∗P<0.05 versus ascorbic acid). Vit. C, ascorbic acid
Figure 4.
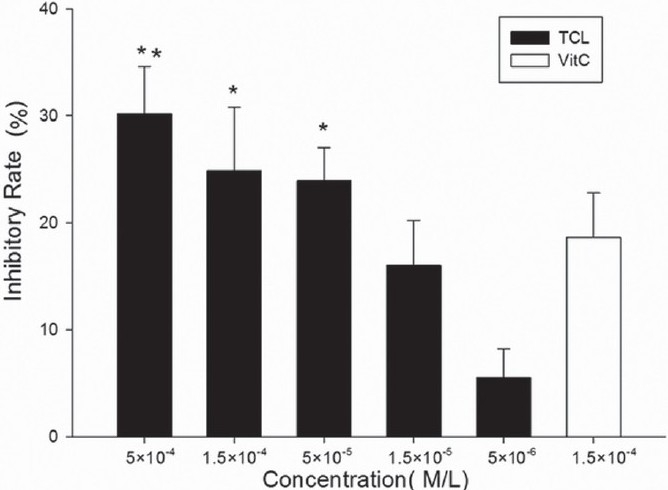
Inhibitory effect of TCL on RBCs autoxidative hemolysis (∗∗P<0.01 versus ascorbic acid, ∗P<0.05 versus ascorbic acid). Vit. C, ascorbic acid
DPPH test showed that TCL is a strong DPPH free-radical scavenger and even at 1.5×10-6 M, it showed inhibitory effect (13.3%±6.7%, P<0.05 vs. control). An obvious dose-dependent manner was observed with calculated IC50=2.4×10-5 M. Ascorbic acid showed similar potency [Figure 5].
Figure 5.
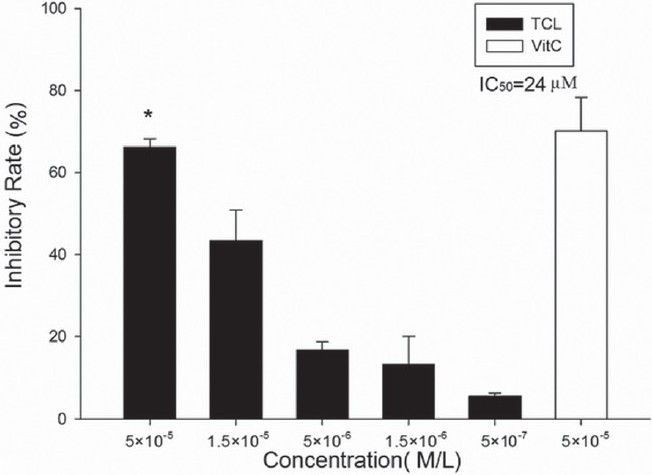
Effect of TCL on DPPH free radical (∗P>0.05 versus ascorbic acid) Vit. C, ascorbic acid
Sodium azide induced mitochondrial ROS production was suppressed to some degree. The inhibitory rate at 1×10-4 M was only about 26.4%. Ascorbic acid at this concentration showed much higher ROS scavenge ability [Figure 6].
Figure 6.
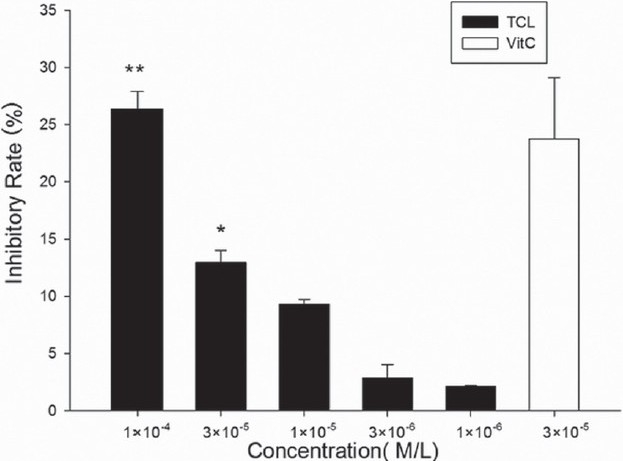
TCL-inhibited sodium azide induced mitochondrial ROS production. Results are expressed as inhibitory rate of DCF formation. (∗∗P<0.05 versus ascorbic acid, ∗P<0.05 versus ascorbic acid). Vit. C, ascorbic acid
Discussion
In recent years, the antioxidant ability of Terminalia chebula Retz has been reported. An aqueous extract significantly reversed t-BHP-induced hepatocyte cytotoxicity in vitro and liver oxidative stress injury in vivo.[25] Several pure compounds such as casuarinin, chebulanin, chebulinic acid, 1,6-di-O-galloyl-b-D-glucose and chebulic acid also showed anti-lipid peroxidation, anti-superoxide radical formation and free-radical scavenging activities.[1,26] However, there are few reports about the biological effect of TCL. To the best of our knowledge, this is the first study reporting the antioxidant activities of TCL.
MDA, one of the products of lipid peroxidation, is widely used for determination of lipid peroxidation.[18] TCL dose dependently inhibited FeSO4/Cys-induced microsomes MDA formation suggesting that TCL might act as a free-radical scavenger. RBC was an excellent model for membrane studies and redox analysis. Significant inhibition of H2O2-induced RBC hemolysis and autoxidative hemolysis revealed that TCL could protect membrane destruction from oxidative injury. The μM level of IC50 and a much higher inhibitory rate than that of ascorbic acid make it a potential agent for further study. It is interesting to note that TCL showed higher potency in H2O2-induced hemolysis than in autoxidative hemolysis. DCFH2-DA is an oxidative stress sensitive fluorescent dye widely used for ROS measurements both in cells and mitochondria.[27] Mitochondrion is both the energy houses and the most important sources of ROS in cells. Inhibition of mitochondrial ROS formation might provide protection for mitochondria from oxidative injury. DPPH reactivity is a popular method for screening the free-radical scavenging ability of compounds or the anti-oxidant activity of plant extracts. Some compounds were active in the DPPH assay but significantly less active in the DCFH system.[28] However, TCL was active in both the DPPH and the DCFH assays.
In summary, our study revealed that TCL, a natural product from Terminalia chebula Retz. was a strong anti-oxidant and free-radical scavenger. The anti-oxidant and free-radical scavenging activity might be one of the mechanisms involved in Terminalia chebula Retz.'s beneficial effect.
Acknowledgments
This study was supported by Hi-Tech Research and Development Program of China (No. 2002AA2Z343B) and State Ethnic Affairs Commission Research Program of China (No. 08XZ03).
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Cheng HY, Lin TC, Yu KH, Yang CM, Lin CC. Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharm Bull. 2003;26:1331–5. doi: 10.1248/bpb.26.1331. [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Oketani H, Singyouchi K, Ohtsubo T, Kihara M, Shibata H, et al. Extraction and purification of effective antimicrobial constituents of Terminalia chebula RETS. against methicillin-resistant Staphylococcus aureus. Biol Pharm Bull. 1997;20:401–4. doi: 10.1248/bpb.20.401. [DOI] [PubMed] [Google Scholar]
- 3.Malekzadeh F, Ehsanifar H, Shahamat M, Levin M, Colwell RR. Antibacterial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori. Int J Antimicrob Agents. 2001;18:85–8. doi: 10.1016/s0924-8579(01)00352-1. [DOI] [PubMed] [Google Scholar]
- 4.Saleem A, Husheem M, Härkönen P, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol. 2002;81:327–36. doi: 10.1016/s0378-8741(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 5.Murali YK, Anand P, Tandon V, Singh R, Chandra R, Murthy PS. Long-term effects of Terminalia chebula Retz. on hyperglycemia and associated hyperlipidemia, tissue glycogen content and in vitro release of insulin in streptozotocin induced diabetic rats. Exp Clin Endocrinol Diabetes. 2007;115:641–6. doi: 10.1055/s-2007-982500. [DOI] [PubMed] [Google Scholar]
- 6.Reddy DB, Reddy TC, Jyotsna G, Sharan S, Priya N, Lakshmipathi V, et al. Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J Ethnopharmacol. 2009;124:506–12. doi: 10.1016/j.jep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Kamaraj C, Rahuman AA, Bagavan A, Elango G, Rajakumar G, Zahir AA, et al. Evaluation of medicinal plant extracts against blood-sucking parasites. Parasitol Res. 2010;106:1403–12. doi: 10.1007/s00436-010-1816-z. [DOI] [PubMed] [Google Scholar]
- 8.Ponnusankar S, Pandit S, Venkatesh M, Bandyopadhyay A, Mukherjee PK. Cytochrome P450 Inhibition Assay for Standardized Extract of Terminalia chebula Retz. Phytother Res. 2010;25:151–4. doi: 10.1002/ptr.2993. [DOI] [PubMed] [Google Scholar]
- 9.Sun FY, Chen XP, Wang JH, Qin HL, Yang SR, Du GH. Arjunic acid, a strong free radical scavenger from Terminalia arjuna. Am J Chin Med. 2008;36:197–207. doi: 10.1142/S0192415X08005709. [DOI] [PubMed] [Google Scholar]
- 10.Lu PP, Liu XK, Li XC. Studies on the chemical constituents of fruits of Terminalia Chebula Retz. J Shanghai Med Univ. 1991;18:233–5. [Google Scholar]
- 11.Yang JR, Sun FY, Li ZH, Du GH, Qin HL. Chemical constitutents from Terminalia Chebula Retz. Nat Prod Res Dev. 2008;20:450–1. [Google Scholar]
- 12.Cederbaum AI, Cohen G. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press; 1986. Microsomal oxidation of hydroxyl radical scavenging agents; pp. 81–7. [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Rodriguez J, Di Pierro D, Gioia M, Monaco S, Delgado R, Coletta M, et al. Effects of a natural extract from Mangifera indica L, and its active compound, mangiferin, on energy state and lipid peroxidation of red blood cells. Biochim Biophys Acta. 2006;1760:1333–42. doi: 10.1016/j.bbagen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Pallotti F, Lenaz G. Isolation and subfractionation of mitochondria from animal cells and tissue culture lines. Methods Cell Biol. 2001;65:1–35. doi: 10.1016/s0091-679x(01)65002-7. [DOI] [PubMed] [Google Scholar]
- 16.Cai YJ, Fang JG, Ma LP, Yang L, Liu ZL. Inhibition of free radical-induced peroxidation of rat liver microsomes by resveratrol and its analogues. Biochim Biophys Acta. 2003;1637:31–8. doi: 10.1016/s0925-4439(02)00174-6. [DOI] [PubMed] [Google Scholar]
- 17.Palozza P, Krinsky NI. Beta-Carotene and alpha-tocopherol are synergistic antioxidants. Arch Biochem Biophys. 1992;297:184–7. doi: 10.1016/0003-9861(92)90658-j. [DOI] [PubMed] [Google Scholar]
- 18.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 19.Tavazzi B, Amorini AM, Fazzina G, Di Pierro D, Tuttobene M, Giardina B, et al. Oxidative stress induces impairment of human erythrocyte energy metabolism through the oxygen radical-mediated direct activation of AMP-deaminase. J Biol Chem. 2001;276:48083–92. doi: 10.1074/jbc.M101715200. [DOI] [PubMed] [Google Scholar]
- 20.Fei LM, Pan HZ, Zhang ZN. The toxicity of maltol and its effect on antioxidative enzyme activities of erythrocytes in mice. Chin Pharm Bull. 1990;6:26–9. [Google Scholar]
- 21.Kang HM, Saltveit ME. Antioxidant enzymes and DPPH-radical scavenging activity in chilled and heat-shocked rice (Oryza sativa L.) seedlings radicles. J Agric Food Chem. 2002;50:513–8. doi: 10.1021/jf011124d. [DOI] [PubMed] [Google Scholar]
- 22.Hou WC, Lin RD, Cheng KT, Hung YT, Cho CH, Chen CH, et al. Free radical-scavenging activity of Taiwanese native plants. Phytomedicine. 2003;10:170–5. doi: 10.1078/094471103321659898. [DOI] [PubMed] [Google Scholar]
- 23.Degli Esposti M. Measuring mitochondrial reactive oxygen species. Methods. 2002;26:335–40. doi: 10.1016/S1046-2023(02)00039-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen XP, Du GH. High-throughput screening for reactive oxygen species scavengers targeting mitochondria. Methods Find Exp Clin Pharmacol. 2008;30:255–60. doi: 10.1358/mf.2008.30.4.1166222. [DOI] [PubMed] [Google Scholar]
- 25.Lee HS, Won NH, Kim KH, Lee H, Jun W, Lee KW. Antioxidant effects of aqueous extract of Terminalia chebula in vivo and in vitro. Biol Pharm Bull. 2005;28:1639–44. doi: 10.1248/bpb.28.1639. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Jung SH, Yun BS, Lee KW. Isolation of chebulic acid from Terminalia chebula Retz.and its antioxidant effect in isolated rat hepatocytes. Arch Toxicol. 2007;81:211–8. doi: 10.1007/s00204-006-0139-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Zhong Z, Xu Z, Chen L, Wang Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic Res. 2010;44:587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 28.Takamatsu S, Hodges TW, Rajbhandari I, Gerwick WH, Hamann MT, Nagle DG. Marine natural products as novel antioxidant prototypes. J Nat Prod. 2003;66:605–8. doi: 10.1021/np0204038. [DOI] [PMC free article] [PubMed] [Google Scholar]


