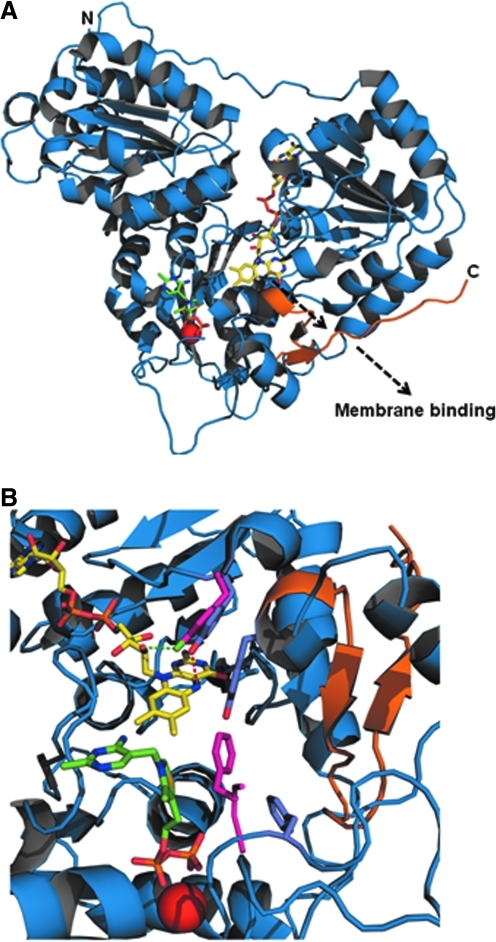
FIG. 5.
EcPOX structure and alignment. (A) Full-length structure of EcPOX monomer shown in cartoon, with TPP and FAD shown as sticks, and Mg2+ shown as a red sphere. FAD and TPP are colored yellow and green, respectively. The residues that make up the C-terminal peptide (550–572) are colored orange. The N and C-terminus are labeled N and C, respectively. The dashed black arrow indicates the release of the C-terminal peptide from the surface triggered by FAD reduction. (B) Overlay of the full-length (1–572) and C-terminal truncated (1–549) structures of EcPOX. Coloring is the same as in (A). The side chains for Y278 and F465 are shown in the overlay as sticks and colored in light purple and bright pink for the full-length and truncated structures, respectively. The side chain for Y549 is also shown but is only seen in the full-length structure. The structures were modeled using PyMol and PDB accession codes 3EY9 (full-length structure) and 3EYA (truncated structure). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).