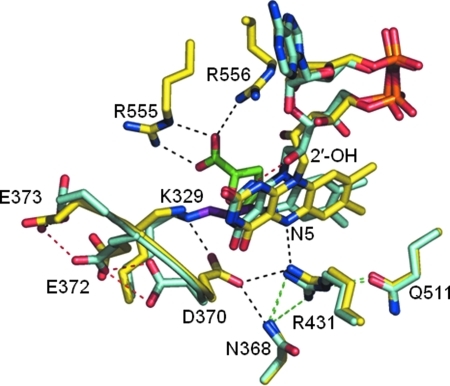
FIG. 8.
Structural differences between THFA-bound and PPG inactivated PutA. THFA-bound (yellow) and PPG-inactivated (teal) PRODH structures were aligned. Shown are the bound FAD cofactors and key active site residues. THFA is shown in green, and the three-carbon link between Lys329 and the FAD N5 is shown in purple for the PPG-inactivated PRODH structure. Hydrogen bonds that are observed in both structures are in green. Unique hydrogen bond interactions in the THFA-bound structure are shown in black, and hydrogen bond interactions that are only observed in the PPG-inactivated structure are shown in red. Models were generated using PyMol and the PDB codes for THFA-bound (1TIW) and for the PPG-inactivate enzyme (3ITG). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).