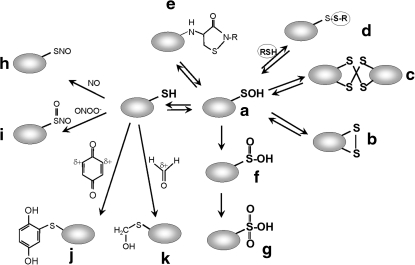
FIG. 1.
Post-translational cysteine modifications involved in redox-sensing mechanisms. Cysteine can be reversibly oxidized by ROS to the unstable Cys sulfenic acid (R-SOH; a), which can then form an intramolecular disulfide (b), an intermolecular disulfide (c), an S-thiolated adduct with a LMW thiol (RSH) (d), or a cyclic sulfenamide with a polypeptide backbone amide (e). Irreversible Cys modifications include overoxidation to Cys sulfinic (R-SO2H) (f) and sulfonic acids (R-SO3H) (g). RNS, such as nitric oxide (NO) or peroxynitrite (ONOO−), modify Cys by S-nitrosothiol formation (R-SNO) (h) or S-nitrothiol formation (R-SNO2) (i). RES with partial positively charged carbon centers (δ+), such as quinones or aldehydes, lead to thiol-(S)-alkylation of cysteine, resulting in quinone-S-adducts (j) by benzoquinone or S-hydroxymethylthiol modification by formaldehyde (k). This figure is modified from (71).