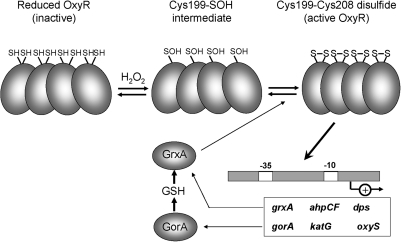
FIG. 2.
The disulfide-switch model for transcriptional activation of OxyR in Escherichia coli. OxyR responds to hydrogen peroxide (H2O2) in E. coli and other bacteria. The conserved C199 and C208 residues are essential for redox-sensing activity. C199 is initially oxidized to the sulfenic acid intermediate that rapidly reacts further to form an intramolecular disulfide with Cys208. Oxidized OxyR binds as a tetramer to promoter regions to activate transcription of genes encoding proteins with antioxidant functions, including GrxA, which is involved in the reduction of oxidized OxyR.