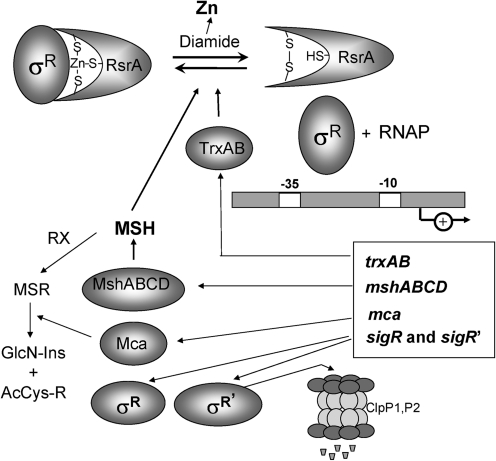
FIG. 3.
Redox regulation of the Zn-binding antisigma factor RsrA of Streptomyces coelicolor. RsrA is a zinc-binding, redox-sensitive anti-σ factor that sequesters σR under reducing conditions. Diamide treatment causes intramolecular disulfide-bond formation in RsrA, resulting in the release of Zn(II) and active σR. Free σR activates transcription of σR regulon genes, including trxBA and trxC (thioredoxin and thioredoxin reductase), mshA (a glycosyltransferase catalyzing the first step in MSH synthesis), and mca (mycothiol-S-conjugate amidase). Collectively, these thiol-reducing systems function in reduction of oxidized RsrA and restore the thiol redox balance. σR and the unstable σR′protein (with a destabilizing N-terminal extension) are positively autoregulated (see text).