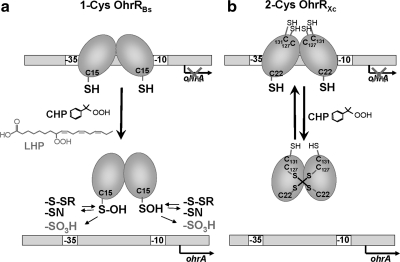
FIG. 5.
Redox-sensing mechanisms of the 1-Cys and 2-Cys type OhrR family proteins. OhrR controls the OhrA thiol-dependent peroxidase and contains one conserved reactive Cys15 residue in B. subtilis and three Cys residues (C22, C127, and C131) in X. campestris. (a) The 1-Cys OhrR protein of B. subtilis is initially oxidized by CHP to the Cys sulfenic acid, which further undergoes reversible S-thiolation with Cys and BSH in vivo and may also generate a cyclic sulfenamide when thiols are depleted. In the presence of the strong oxidant LHP, C15 is irreversibly overoxidized to the sulfinic and sulfonic acids. (b) The 2-Cys OhrR protein of X. campestris is regulated by intersubunit disulfide formation between C22 and C127’ of opposing subunits. Both Cys residues are essential for redox sensing in vivo, as shown by mutagenesis (73).